neuroscience exam 2 flashcards
1/130
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
131 Terms
classical neurotransmitters (3 types + characteristics)
-acetylcholine
-monoamines: catecholamines→ dopamine, norepinephrine and epinephrine & indolamines→ serotonin
-amino acids: glutamate & GABA
-Released by the presynaptic vesicle in "quotas", synthesized in the terminal, and packed in vesicles
semi-classical neurotransmitters
peptides (small proteins). Only difference between classical and semi-classical is that the latter are synthesized in the soma
non-classical neurotransmitters (3 types + characteristics)
-lipids
-nucleosides
-soluble gasses
-Released by the postsynaptic cell, not released in quotas, not packed in vesicles. Still transmit info between neurons, so they are called neurotransmitters
Acetylcholine
classical neurotransmitter. Primary neurotransmitter secreted by axons of the PNS that terminate at muscle cells to control muscle contraction. Primary means by which postsynaptic action of acetylcholine is terminated is by enzymatic degradation.
Botulinum toxin
acetylcholine antagonist, prevents release by terminal buttons (can result in paralyzation). ACh is loaded into vesicles by the vesicle ACh transporter, where it is stored until being released from the presynaptic cell.
synthesis of acetylcholine
Acetylcholine is composed of choline and acetyl coenzyme A. The enzyme choline acetyltransferase (ChAT) is required to produce ACh from the precursors. After being released by the terminal button, ACh is deactivated by the enzyme acetylcholinesterase (AChE), which is present in the postsynaptic membrane. AChE breaks down one molecule of ACh into its two precursors
acetylcholine and its different locations
-dorsolateral pons→ REM sleep
-basal forebrain→ activating cortex, perceptual learning
-medial septum→ electrical rhythms of the hippocampus and modulate its functions
Black widow spider venom
poison that triggers the release of acetylcholine until there is no more acetylcholine to be released anymore
Myasthenia gravis
weakening of neuromuscular function. autoimmune disorder in which an antibody is made for acetylcholine receptors, which makes it hard for acetylcholine to attach. Solution is a drug that increases acetylcholine so that it has more chances to attach
Neostigmine
drug that inhibits activity of acetylcholinesterase so that acetylcholine stays in cleft for a longer time (therefore it has more chances to attach)
acetylcholine receptors (2)
-Nicotinic receptor: ionotropic receptor that is stimulated by nicotine & blocked by curare
-Muscarinic receptor: metabotropic receptor that is stimulated by muscarine and blocked by atropine
monoamines
indoleamines and catecholamines
indolamines
class of amines that includes serotonin
Catecholamines
class of amines that includes dopamine, norepinephrine and epinephrine
synthesis of catecholamines
The precursor molecule is modified slightly, step by step, until it achieves its final shape. Each step is controlled by a different enzyme, which causes a small part of the molecule to be added or taken off
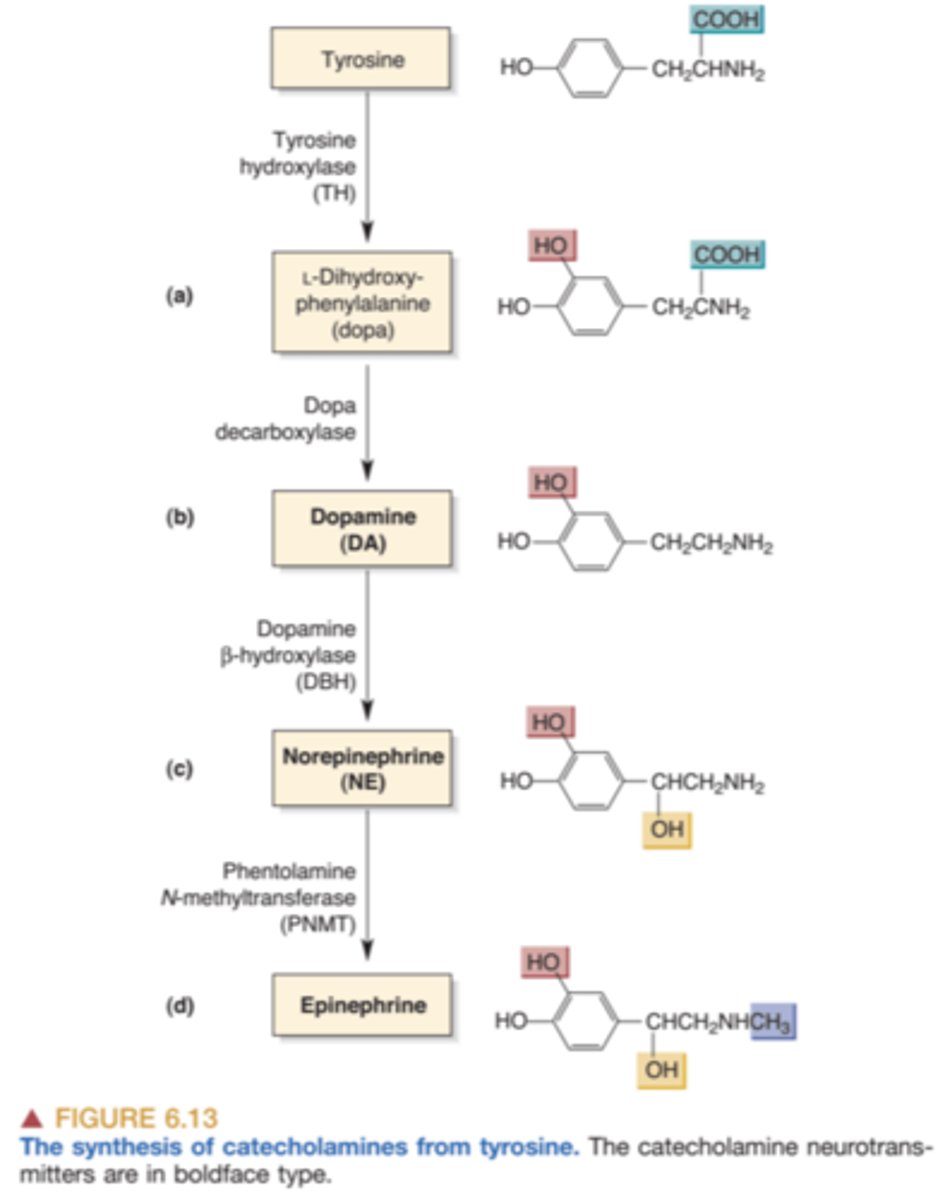
synthesis of catecholamines: tyrosine
Rate limiting enzyme. Activation is associated with phosphorylation which speeds activity. Precursor of dopamine and norepinephrine. Modified by tyrosine hydroxylase and becomes L-Dopa
synthesis of catecholamines: L-Dopa
Precursor. Modified through the activity of the enzyme DOPA decarboxylase and becomes dopamine. Often used to treat Parkinson's disease because of its effect as a dopamine agonist
synthesis of catecholamines: Dopamine β-hydroxylase
converts dopamine to norepinephrine. Only neurons that release norepinephrine contain dopamine β-hydroxylase; however, both dopamine- and norepinephrine-releasing neurons contain tyrosine hydroxylase
dopamine
classical neurotransmitter. concentrated within the vesicles and bound to the inner membrane, released along with the neurotransmitters. produces both excitatory and inhibitory postsynaptic potentials, depending on the postsynaptic receptor
dopamine pathways
-mesocortical system
-mesolimbic system
-nigrostriatal system
-tuberoinfundibular pathway
-thalamus dopamine system
Mesocortical system
system of dopaminergic neurons originating in the ventral tegmental area and terminating in the prefrontal cortex. These neurons have an excitatory effect on the frontal cortex and affect functions such forming short-term memories, planning, and problem-solving
Mesolimbic system
system of dopaminergic neurons originating in the ventral tegmental area and terminating in the nucleus accumbens, amygdala and hippo campus. The nucleus accumbens plays an important role in the reinforcing (rewarding) effects of certain categories of stimuli, including those of drugs that people abuse.
Nigrostriatal system
A system of neurons originating in the substantia nigra (part of the brain related to movement) and terminating in the neostriatum (caudate nucleus and putamen). The neostriatum is an important part of the basal ganglia, which is involved in the control of movement
parkinson's disease
neurological disease characterized by tremors, rigidity of the limbs, poor balance, and difficulty initiating movements, caused by degeneration of the nigrostriatal system.
Tuberoinfundibular pathway
connects the population of dopamine neurons in the arcuate nucleus of the mediobasal hypothalamus (the 'tuberal region') to the median eminence (the 'infundibular region'). Regulates the secretion of prolactin from the anterior pituitary gland.
Thalamus dopamine system
Connect the PAG, ventral mesencephalon, hypothalamic nuclei, lateral parabrachial nucleus to the thalamus. Thalamus filters sensory info, and it has been found that glutamate and dopamine are involved in this process
dopamine receptors
5 types of receptors that are all G-protein coupled metabotropic receptors, and can be excitatory or inhibitory to the postsynaptic neuron. Categorized into 2 main subtypes: D1-like and D2-like
D1-like
-Activation is coupled to increases in cAMP and is typically excitatory. They are coupled to Gs.
-D1
-D5
D2-like
-activation reduces cAMP and is typically inhibitory. They are coupled to Gi/o.
-D2:
-D2Sh: short version. presynaptic autoreceptor, they regulates synthesis, storage and release of dopamine into the synaptic cleft
-D2Lh: long version of D2, may have the classic function of a postsynaptic receptor
-D3
-D4
drugs related to dopamine (4)
apomorphine, chlorpromazine, cocaine, amphetamine
Apomorphine
D2 agonist. blocks dopamine autoreceptors at low doses. At higher doses it blocks postsynaptic receptors as well.
Chlorpromazine
reduces the symptoms of schizophrenia by blocking dopamine D2 receptors associated with hallucination and psychosis
Cocaine and Methylphenidate (Ritalin)
Inhibits the reuptake of dopamine. Helps those with ADHD who have lower than optimum levels of dopamine
Amphetamine
Release dopamine and norepinephrine ( most transporters don't differentiate between the two) & cause the transporter to run in reverse
Norepinephrine
classical neurotransmitter. neuroandrogenic, usually in autonomic NS. The cell bodies of the most important noradrenergic system begin in the locus coeruleus, a nucleus located in the dorsal pons
norepinephrine receptors (3)
-Beta 1: excitatory, Gs
-Alpha 1: excitatory, postsynaptic terminal, Gq
-Alpha 2: inhibitory, autoreceptor, Gi
PNMT
enzyme found primarily in the adrenal medulla that converts norepinephrine (noradrenaline) to epinephrine
epinephrine
classical neurotransmitter. androgenic, found in small group of neurons in brainstem, activity regulated by corticosteroids
AMPT
blocks the activity of tyrosine hydroxylase and thus interferes with the synthesis of catecholamines
Reserpine
interferes with storage of monoamines in synaptic vesicles. If monoamines can't move into vesicles they are either not released or destroyed
Fusaric acid
inhibits activity of enzyme dopamine-ßhydroxylase and thus the production of norepinephrine without affecting the production of dopamine
inactivation of catecholamines (2)
MAO and COMT
Monoamine oxidase (MAO, 2)
-intra-cellular, located in the outer membrane of the mitochondria., play a role in inactivating catecholamines that are free within the nerve terminals and not protected by storage vesicles
-MAO-A: very selective for NE and serotonin
-Moclobemide: blocks the activity of MAO-A, increase noradrenergic tone
-MAO-B: Dopamine. acts on a broad spectrum of phenylethylamines. severe hypertensive crises after ingestion of food containing large amount of tyramine (port wine, cheese, herrings)
-Deprenyl: block activity of MAO-B, increase dopaminergic tone
Catechol-O-methyltransferase (COMT)
found in nearly all cells, acts on extraneuronal catecholamines
Serotonin (5HT)
classical indoleamine neurotransmitter, also called 5-hydroxytryptamine. It exists in different locations around the body, which is why it is known by several different names. involved in mood and pain regulation, and the control of eating, sleep, arousal, and dreaming.
synthesis of serotonin
structure primarily responsible for the production of serotonin is the raphe nuclei. The precursor for serotonin is the amino acid tryptophan. The enzyme tryptophan hydroxylase acts on tryptophan, producing 5-HTP (5-hydroxytryptophan). The enzyme 5-HTP decarboxylase converts 5-HTP to 5-HT (serotonin).
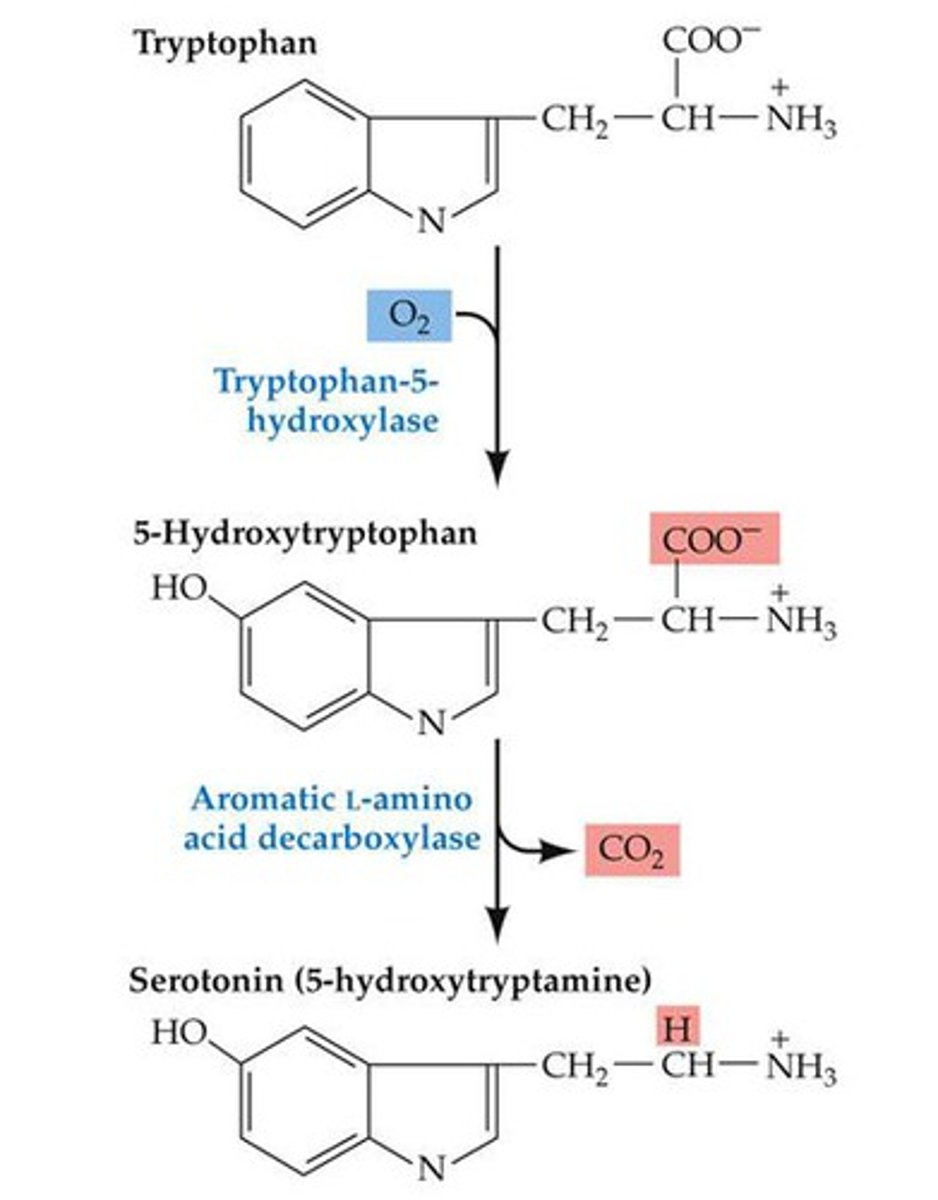
PCPA
drug that inhibits the activity of tryptophan hydroxylase and thus interferes with the synthesis of 5-HT
serotonin receptors (6)
5HT1A, 5HT1B, 5HT1D, 5HT1A-2C, 5HT4, 5HT3
5HT1A
Autoreceptor on dendrites and soma. inhibition of adenylyl cyclase (postsynaptic, Gi)
5HT1B and 5HT1D
Presynaptic autoreceptor, inhibition of adenylyl cyclase (Gi)
5HT2A-2C
Stimulation of phosphoinositide-specific phospholipase C (Gq). Related to the subjective feelings of anxiety & sleep
5HT4
Stimulation of adenylyl cyclase (Gs)
5HT3
Ionophoric. No G protein coupled to it. Related to appetite and eating disorders. Located in the hypothalamus and guts
drugs related to serotonin (4)
-fluoxetine/prozac
-fenfluramine
-lsd
-mdma
Fluoxetine (Prozac)
inhibits the reuptake of 5-HT. Used to treat Depression/OCD
Fenfluramine
stimulates the release of 5-HT. Appetite suppressant used to treat obesity
LSD
stimulates 5HT2A receptors
MDMA ("ecstasy")
Cause the transporter for serotonin to run in reverse (release serotonin) leading to a hallucinogenic effect. Also cause the transporter for norepinephrine to run in reverse (release norepinephrine) leading to an excitatory effect
glutamate
classical neurotransmitter, amino acid. main excitatory neurotransmitter in the brain and spinal cord. Synthesized from glutamine by the enzyme glutaminase in one step. After synthesization, it is stored in vesicles in the presynaptic neurons, from where it's released after an action potential
ionitropic glutamate receptors (3+ cotransmitters)
-NMDA
- glycine, L- and D-serine
-AMPA
-Kainate
NMDA
specialized ionotropic glutamate receptor that controls a calcium channel that is normally blocked by Mg+ ions (Mg+ dissociates from the membrane when it is depolarized); has several other binding sites. Voltage dependent. Calcium serves as a second messenger, binding with—and activating—various enzymes within the cell. One important result of this second messenger system is an alteration in the characteristics of the synapse that provide one of the building blocks of a newly formed memory
Glycine
comes from glial cells. leads to a lot more activation of the receptor
L- and D-serine
L-serine is consumed in the diet and D-serine is made in the body from L-serine. The body uses D- and L-serine to make proteins. D-serine also sends chemical signals in the brain. This might help with schizophrenia and other brain conditions.
AMPA
An ionotropic glutamate receptor that controls a sodium channel. It is stimulated by AMPA and when glutamate attaches to a binding site, it produces EPSP. Fastest response, most common glutamate receptor, and most times it is not voltage dependent
kainate
ionotropic glutamate receptor that controls a sodium channel; stimulated by kainic acid
Metabotropic glutamate receptors
mGluRs. 8 different types, divided into group I, II, and III based on similarity, pharmacology and intracellular signaling mechanisms

drugs related to glutamate (2)
-AP5
-PCP
AP5 (2-amino-5-phosphonopentanoate)
A drug that blocks the glutamate binding site on NMDA receptors.
PCP
Phencyclidine; a drug that binds with the PCP binding site of the NMDA receptor and serves as an indirect antagonist (when it attaches to its binding site, calcium ions cannot pass through the ion channel)
GABA
amino acid, classical neurotransmitter. Inhibitory neurotransmitter with widespread distribution throughout the brain and spinal cord. Produced from glutamic acid by the action of an enzyme (glutamic acid decarboxylase, or GAD).
GABA receptors
-Divided into 3 classes: GABAa, GABAb and GABAc
-GABAa, GABAc: GABA-gated chloride ion-conducting channels. Varieties of GABAa are found all over the CNS, and GABAc receptors are primarily found in the retina
-GABAb: G-protein coupled receptors. Inhibitory, metabotropic autoreceptor
drugs related to GABA (2)
-benzodiazepine
-alcohol
Benzodiazepine
anxiolytics, or "anxiety-dissolving" drugs, and are used to reduce symptoms of anxiety, reduce seizure activity, and produce muscle relaxation
alcohol
Indirect GABA receptor agonist (among other mechanisms). Effects are sedation, memory impairment, muscle relaxation
neuropeptides
semi-classical neurotransmitter. small proteins produced by neurons that act on G protein-coupled receptors and are responsible for slow-onset, long-lasting modulation of synaptic transmission
endogenous opioid
class of peptides secreted by the brain that act as opiates. Reduce pain because they have direct effects on the brain through their actions in the endogenous opioid system.
Naloxone
blocks opiate receptors
lipids
non-classical neurotransmitter. retrograde. Lipid neurotransmitters appear to be synthesized on demand; that is, they are produced and released as needed and are not stored in synaptic vesicles.
Endocannabinoid
lipid, endogenous ligand for the cannabinoid receptors, which also binds with THC, the active ingredient of marijuana
Anandime
first cannabinoid to be discover and probably the most important one
THC
active ingredient in marijuana; activates CB1 receptors in the brain
Nucleosides
non-classical neurotransmitter. a compound (e.g., adenosine or cytidine) commonly found in DNA or RNA
Adenosine
combination of ribose and adenine; serves as a neuromodulator in the brain
caffeine
blocks adenosine receptors, thus more norepinephrine is released
soluble gasses
non-classical neurotransmitter. retrograde. Nitric oxide and nitric oxide synthase
Nitric oxide
gas produced by cells in the nervous system; used as a means of communication between cells. non-classical neurotransmitter
How do indoleamines and catecholamines differ?
Catecholamines (dopamine, norepinephrine and epinephrine) are synthesized from tyrosine. Indolamines (serotonin and tryptamine) are synthesized from tryptophan
What is a rate limiting enzyme?
enzyme of which the activity determines the overall rate of a metabolic pathway
What disease is MPTP made to model?
Parkinson's disease
Why would you take beta blockers?
Beta blockers block the release of the stress hormones adrenaline and noradrenaline. They are widely prescribed for angina, heart failure and some heart rhythm disorders, and to control blood pressure.
Experimental ablation
removal or destruction of a portion of the brain of a laboratory animal; presumably, the functions that can no longer be performed are the ones the region previously controlled
Excitotoxic lesion
brain lesion produced by intracerebral injection of an excitatory amino acid, such as kainic acid. The amino acid kills neurons by stimulating them to death
6HD
chemical that is selectively taken up by axons and terminal buttons of noradrenergic or dopaminergic neurons and acts as a poison, damaging or killing them. The chemical destroys neural cell bodies in the vicinity but spares axons that belong to different neurons that happen to pass nearby. This selectivity permits the investigator to determine whether the behavioral effects of destroying a particular brain structure are caused by the death of neurons located there or by the destruction of axons that pass nearby
Stereotaxic surgery
Brain surgery using a stereotaxic apparatus to position an electrode or cannula in a specified position of the brain. Uses an atlas (collection of drawings of sections of the brain with measurements that provide coordinates for stereotaxic surgery made in relation to the bregma/front of head) and apparatus (includes a head holder, a holder for an electrode or cannula, and a calibrated mechanism that moves the electrode/cannula holder in measured distances)
histological methods
methods of fixing tissue, slicing tissue, and staining. Done after producing a brain lesion and observing its effects on an animal's behavior so that researchers can observe the brain under the microscope and see the location of the lesion
fixative
A chemical such as formalin; used to prepare and preserve body tissue. It protects the brain from autolytic (self dissolving) enzymes that break down tissue and decomposition by bacteria or molds. Fixatives cross-link proteins to strengthen the very soft and fragile brain tissue and kill any microorganisms that might destroy it. Fixative might not get inside the brain fast enough, so then it might start to rot. To prevent this, we do perfusion
perfusion
process by which an animal's blood is replaced by fluid (dilute saline solution and then dilute fixative solution) in preparing the brain for histological examination. Gets rid of the protein in the blood
Microtome
instrument that produces very thin slices of tissues. Contains a knife, a platform on which to mount the tissue, and a mechanism that advances the knife (or the platform) the correct distance after each slice so that another section can be cut. In most cases, the platform includes an attachment that freezes the brain to make it hard enough to be cut into thin sections
Immunocytochemical methods
neurochemical histological method that uses radioactive antibodies or antibodies bound with a dye molecule to indicate the presence of particular proteins or peptides. antibodies attach themselves to their antigen. When the investigator examines the slices with a microscope (under light of a particular wavelength in the case of fluorescent dyes), they can see which parts of the brain—even which individual neurons—contain the antigen.Sometimes we want to know about functional proteins, certain receptors, etc. we use this method for that. Used for tracing efferent axons
in situ hybridization
when we want to evaluate a level of protein/ expression of RNA. more RNA means that we have more protein, thus more function. Generally talking this correlation does exist, but it is not always true. You are not measuring the protein itself. The production of radioactive RNA complementary to a particular messenger RNA in order to detect the presence of the messenger RNA.