Reactions of Carboxylic Acids
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
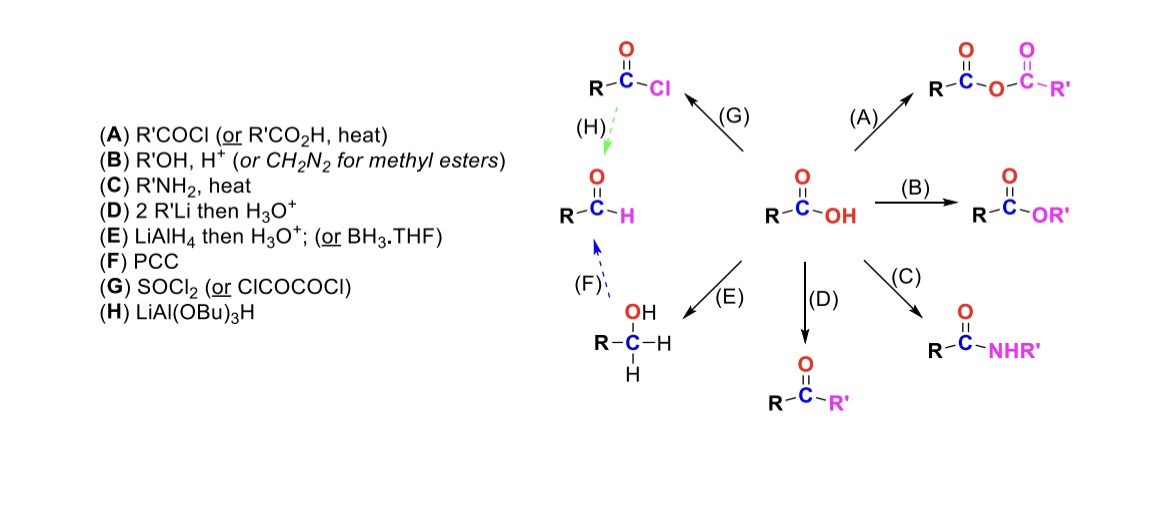
RCOCl (or RCO2H, heat)
Carboxylic acid reacts with an acid chloride to form an anhydride (OH is substituted w/ RCO2)
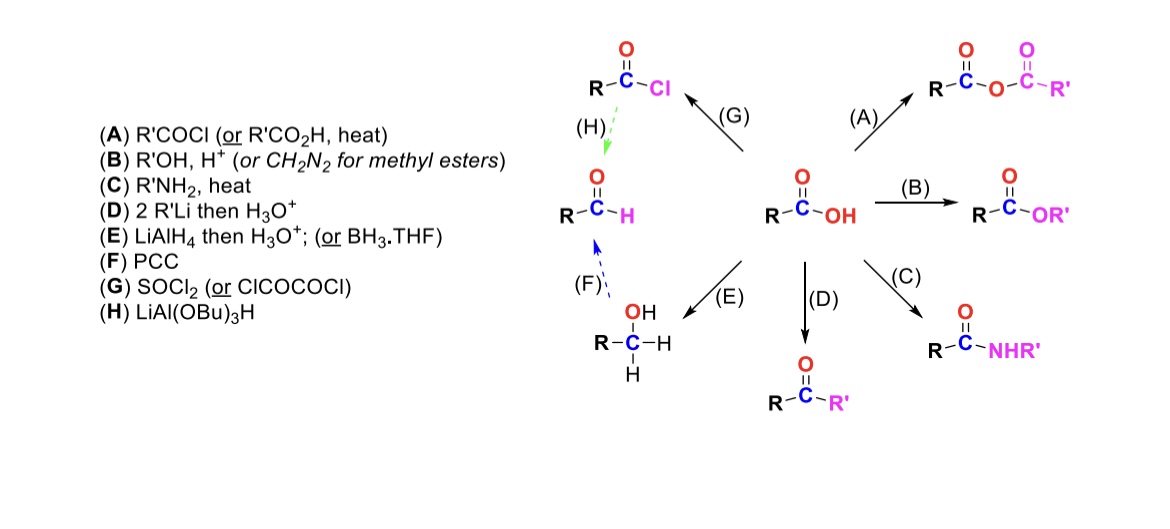
R-OH, H+ (or CH2N2 for methyl esters)
Carboxylic acid reacts with alcohol to form an ester (OH is substituted with OR)
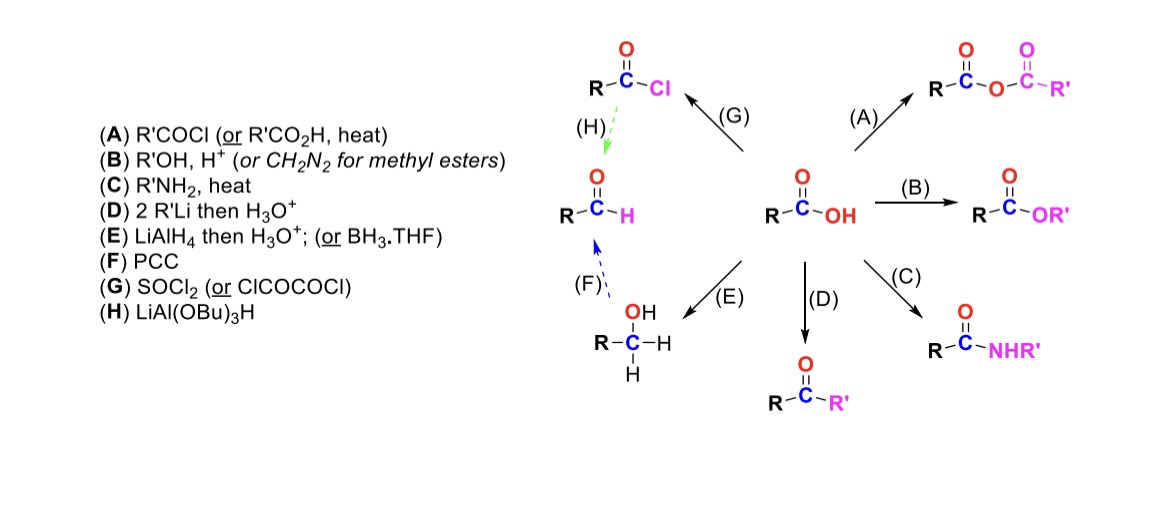
R-NH2, heat
Carboxylic acid reacts with amine to form an amide (OH is substituted w/ NHR)
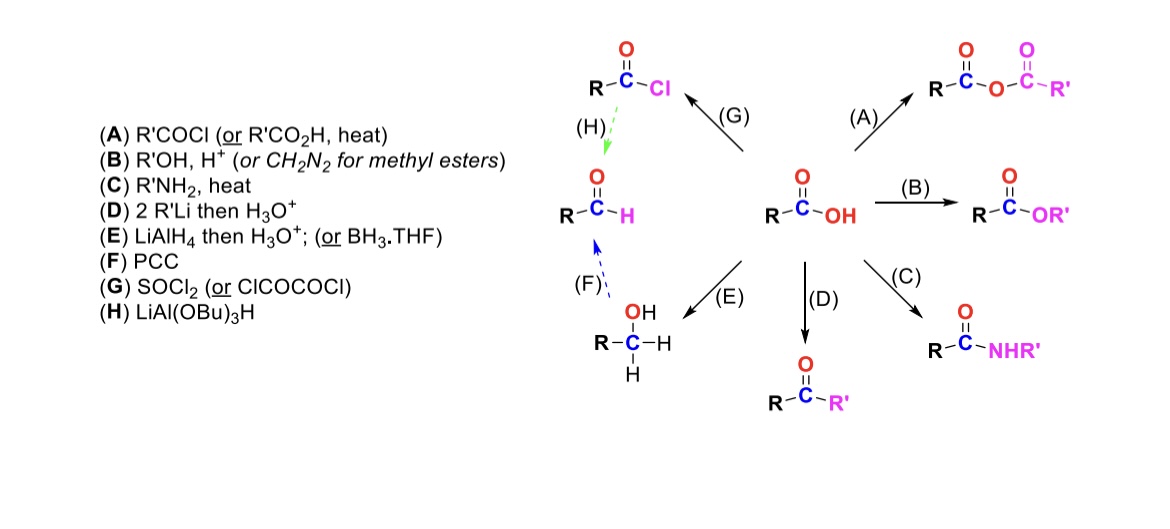
2 eq. R-Li, then H3O+
Carboxylic acid is turned into a ketone (OH is substituted w/ an R group)
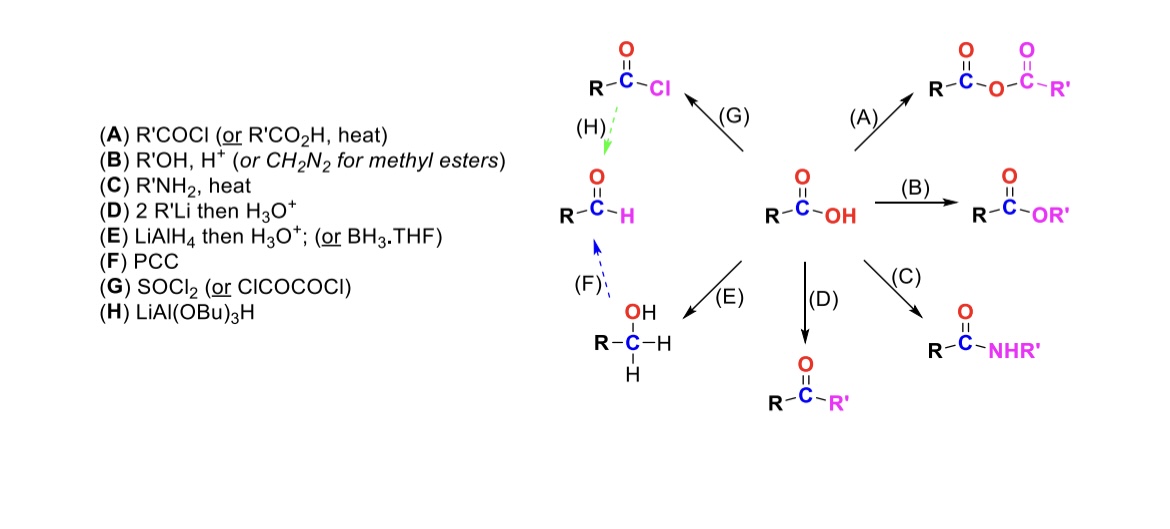
LiAlH4, then H3O+ (or BH3, THF)
Carboxylic acid is reduced into a primary alcohol
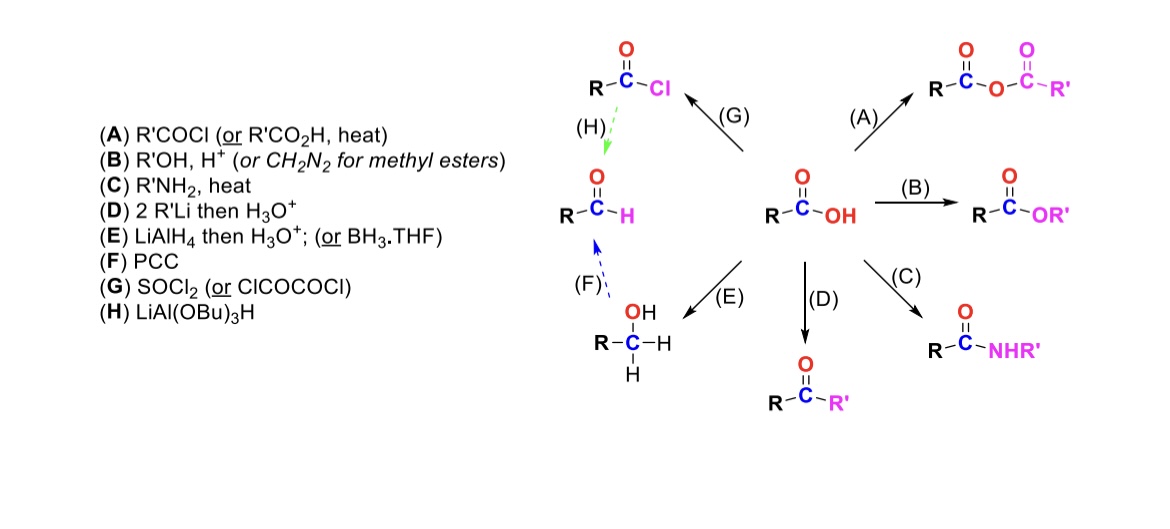
PCC
Primary alcohol is turned into an aldehyde
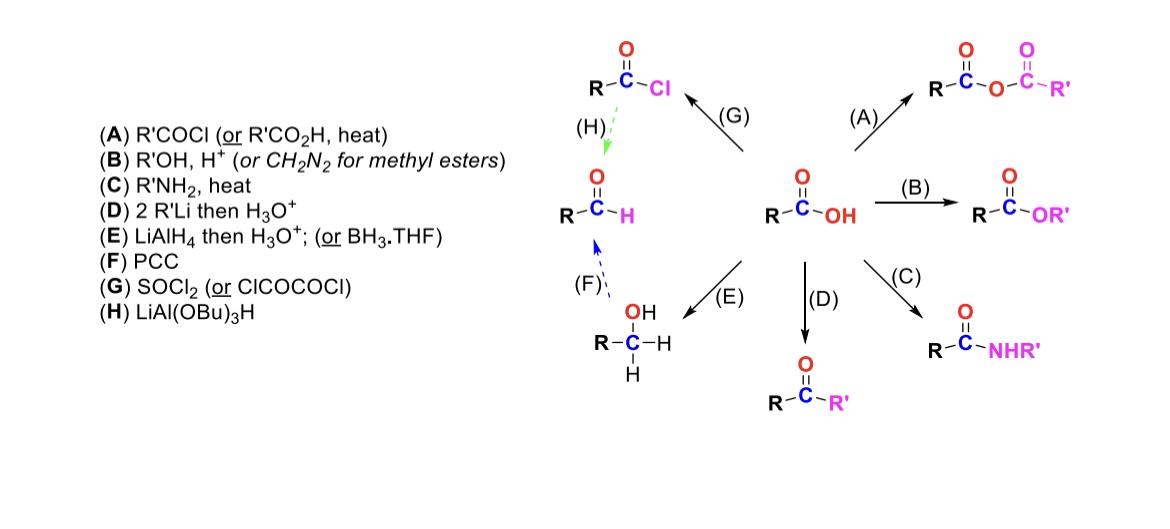
SOCl2
Carboxylic acid is turned into an acid chloride (OH is substituted w/ Cl)
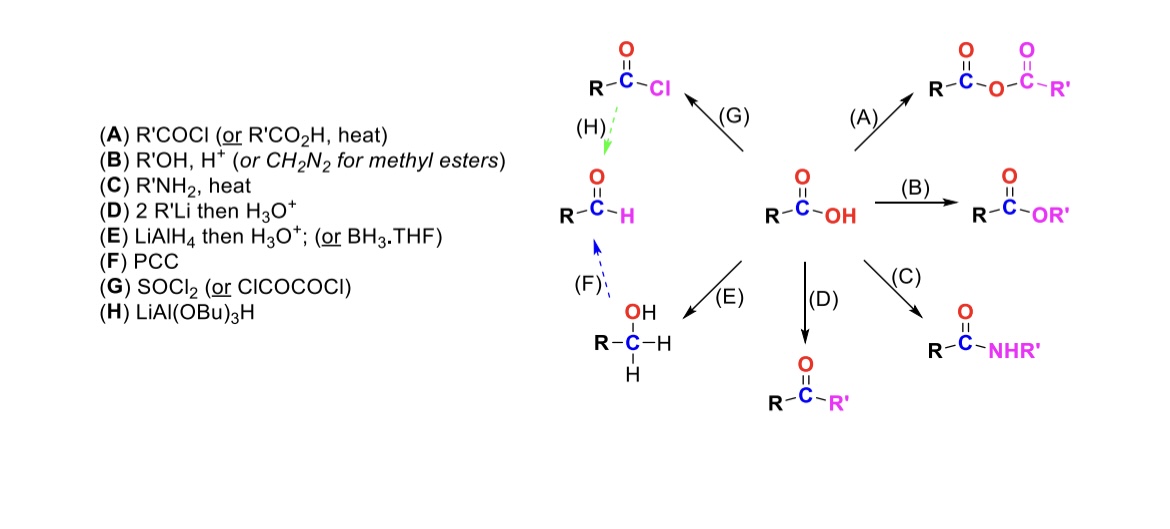
LiAlH(OBu)3
Acid chloride is turned into an aldehyde