Drugs and the Immune System Lecture
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Non-specific or general immunosuppression
Suppress both T and B cell responses to antigens
X-radiation: affects DNA within cells; ionization of water and formation of toxic reactive oxygen and hydroxyl radicals; affects cell division
Used to prolong the graft survival in lab rodents
Corticosteroids
Immunosuppressive and anti-inflammatory agents
Lab rodents and humans are more sensitive to the immune-suppressive effects of corticosteroids than major domestic mammals.
Stimulate the production of excess IkB-alpha and also block NF-kB-mediated inflammatory gene expression.
Mechanism of glucocorticoids
Glucocorticoids pass through the cell membrane and bind to cytoplasmic glucocorticoid receptors, displacing heat shock protein (HSP) molecules. The combination of steroid and receptor then passes into the nucleus where binding to steroid response elements (SRE) within the promoter either initiates gene expression (transactivation) or blocks the ability of pro-inflammatory signalling molecules (such as NF-κB) from binding to their target site, thus inhibiting gene transcription (transrepression).
The effects of corticosteroids on the immune system
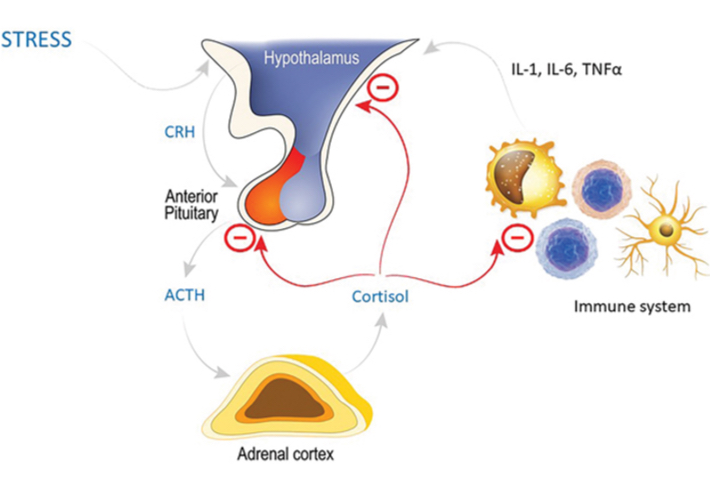
The HPA axis involves release of corticotropin-releasing hormone (CRH) from the hypothalamus acting on the anterior pituitary to stimulate production of ACTH, which in turn stimulates the release of cortisol from the adrenal cortex. Cortisol has a negative feedback loop on both the hypothalamus and anterior pituitary. In addition, there is interaction with the immune system, with pro-inflammatory cytokines promoting the release of CRH, but cortisol having a suppressive effect on the immune system and downregulating synthesis of those pro-inflammatory mediators, in an additional negative feedback loop.
Glucocorticoid use
Small animals: prednisolone/methyl prednisolone
Large animals: betamethasone and dexamethasone
Side effects: Cushing’s syndrome and increased susceptibility to infection (taper the dose)
Cytotoxic drugs
Inhibit cell division and act on nucleic acid synthesis
Azathioprine: affects only proliferating but not resting cells; possesses significant anti-inflammatory action. Hence it is a favored drug for immune-mediated skin diseases.
Beneficial in the control o allograft rejection but it is ineffective in xenograft rejection
Cyclophosphamide: toxic for both resting and dividing immunocompetent cells. Impairs T and B cell responses and suppress macrophage function.
Beneficial in the treatment of lymphoid neoplasia and immune-mediated skin diseases
Methotrexate: used in cancer and immune-mediated diseases. Suppress antibody synthesis
Side effects: bone marrow suppression
Selective immunosuppression
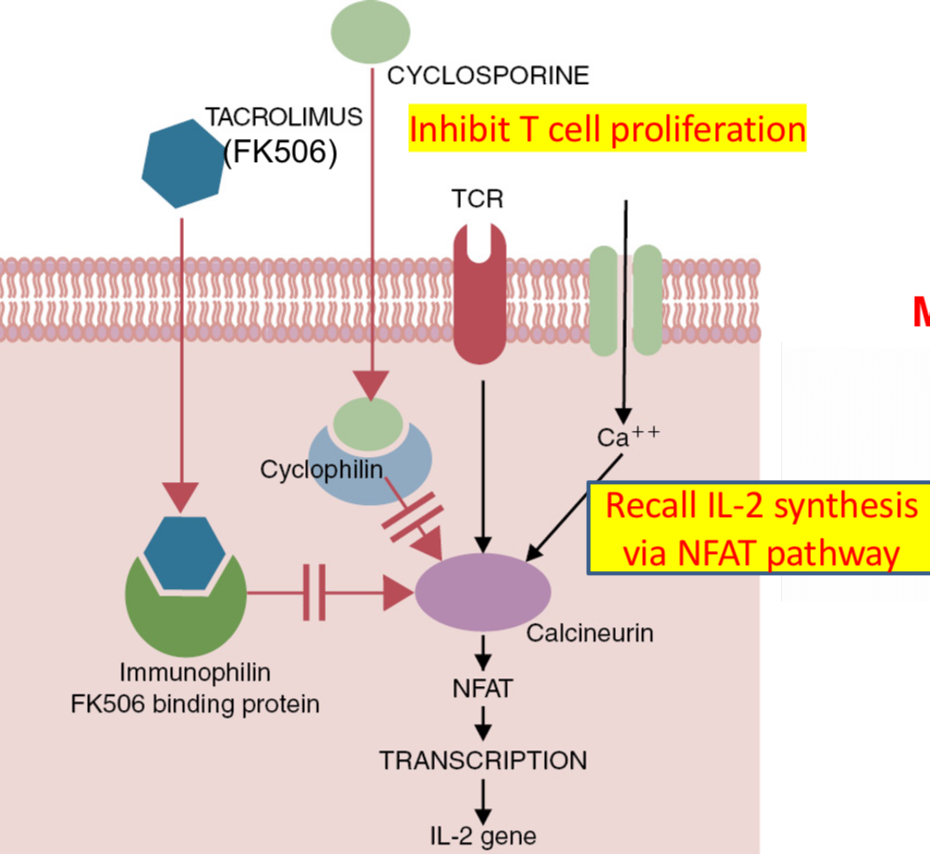
Cyclosporine: a polypeptide derived from fungus Tolypocladium inflatum consists of 11 amino acids arranged in a circle.
Binds cyclophillin and calcineurin, a serine / threonine phosphatase. Cyclosporine inhibits calcineurin and prevent the production of cytokines (immunosuppressant- drug to prevent graft rejection)
Widely used in transplantation; inhibits the production of IL-2 and IFN-g by T cells.
Blocks IFN-g-induced expression of MHC class I
Combination of cyclosporine and corticosteroids proved beneficial to enhance the survival of allografts.
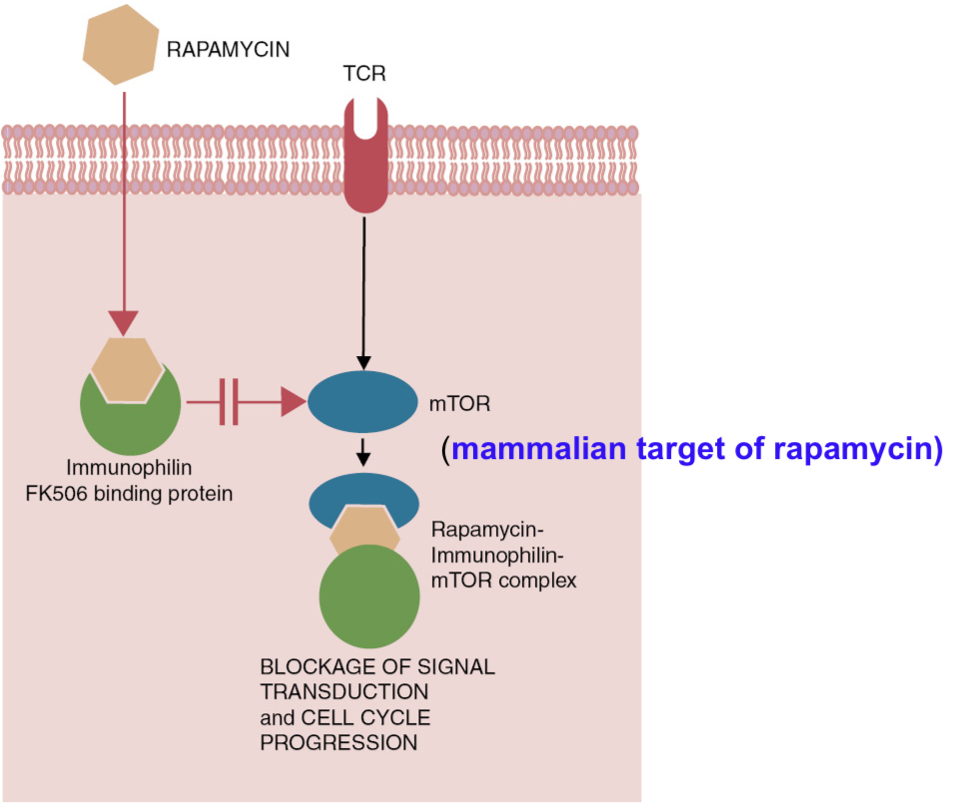
Selective immunosuppression - Rapamycin inhibits lymphocyte proliferation by blocking mTOR pathway
The mode of action of rapamycin. This drug blocks activation of the aptly named mTOR (mammalian target of rapamycin). As a result numerous cell functions are blocked, including gene activation pathways and cell cycle progression. Rapamycin: inhibitor of cellular enzyme called mammalian target of rapamycin (mTOR; serine/threonine protein kinase required for cell proliferation)
Macrolide antibiotic rapamycin (sirlimus, is from bacterium, Streptomyces hygroscopicus) inhibits serine kinase called mammalian target of rapamycin (mTOR). mTOR pathway plays a critical role in regulating T cell activation by integrating the signals received from specific antigen, costimulatory receptors, cytokines and directing T cell differentiation into effector, regulatory and memory pathways. mTOR also acts on non dividing macrophages and dendritic cells by associating with MyD88 pathway; activates IFN regulatory factors and inhibits caspase-1.
Acts on macrophages and DCs; enhances IL-12 and nitric oxide synthesis and inhibits IL-10 leading to Th1 or Th17-mediated inflammation.
Inhibits B and T cell proliferation by blocking stimulatory signals from IL-2, Il-4 and IL-6.
Enhances Treg cell production and promotes tolerance.
Acts synergistically with calcineurin inhibitors but superior to cyclosporine in preventing allograft and xenograft rejections in humans.
Blocks endothelial cell and fibroblast proliferation and hence, it can prevent graft vascular disease but it can inhibit wound healing.
Mice: increases the life span by acting possibly as a dietary restriction mimetic.
Toxicity is severe in dogs: ulceration, vasculitis, vomiting, anorexia etc.
Depletion of T lymphocytes
Antilymphocyte serum (ALS) specific for T lymphocytes: depletes T cells; suppress CMI response. Tested with variable results in transplantation (side effects)
Anti-CD3 monoclonal antibody: proved effective in preventing graft rejections; can also induce tolerance (mechanisms are complex)
Anti-IL-2R antibody: attacks only activated lymphocytes - binds alpha chain of IL-2R and thus prevents lymphocyte activation; help prevent renal graft rejections (fewer side effects than ALS)
Anti-CD4 and anti-CD8 antibodies: tested in canines for renal grafts; proved effective; both must be administered together
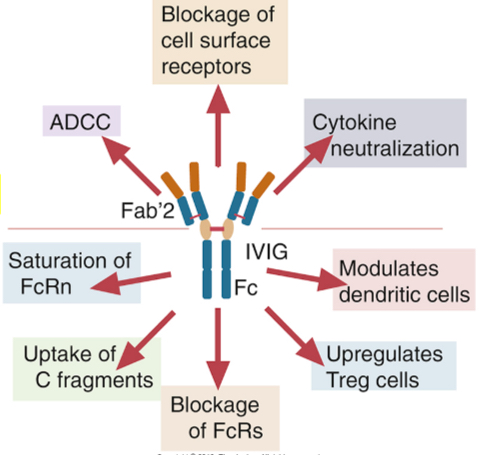
IV immunoglobulin therapy (IVIG) for autoimmune/immune-mediated inflammatory diseases
The major component of IVIG preparations is the serum IgG antibodies, which is pooled from thousands of donors
Administer as anti-inflammatory agent (humans - FDA approved; and dogs)
A few proposed mechanisms for IVIG for autoimmune/immune-mediated inflammatory diseases
Complete with FcRn which functions in adults to protect antibodies from catabolism, resulting in reduced half-lives of pathogenic antibodies
Promote Treg cells (TGF-beta, IL-10)
Block binding of immune complexes formed by pathogenic antibodies to FcyR on macrophages
Promote expression of FcyRII B (inhibitory receptors on B cells and myeloid cells (FcyRIIB expression on B cells shuts down autoantibody-producing B cells)
Bacteria and bacterial products as immune stimulants
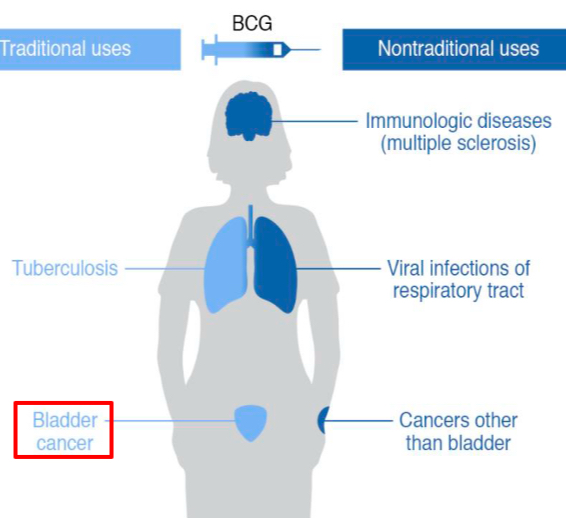
Bacillus Calmette-Guerin (BCG) vaccine - prepared from Mycobacterium bovis: enhances B and T cell responses; tested in the treatment of equine sarcoidosis and ocular squamous cell carcinoma
Unmethylated CpG nucleotides in the bacterial DNA: bind TLR9, and activate APCs, and trigger Th1 response via IL-12 production. Unmethylated CpG motifs are prevalent in bacterial but not vertebrate genomic DNAs. Oligodeoxynucleotides (ODN) containing CpG motifs activate host defense mechanisms leading to innate and acquired immune responses
Act as potent adjuvants when administered with antigens (increase the efficacy of vaccines) or immune stimulants when administered alone
Levamisole
Anthelmintic stimulates T cell responses to antigens
Enhances IFN, CTL activity and stimulates phagocytic activity; beneficial in chronic infections and neoplastic diseases
Vitamins and mineral
Vitamin A metabolites (retinoic acid): differentiation of T cell subsets, modulate the imbalance of Th17 and T reg cells
Vitamin D promotes the production of antimicrobial peptide cathelicidin in macrophages and DCs
Vitamin E and selenium affect immune responses and increase disease resistance (poultry, pigs, and lab animals)
Vitamin E promotes phagocyte function and B cell proliferation
Cytokines
IL-1, IL-2, IL-12, CSF, and IFNs and have been tested in animals, but side effects are always an issue with cytokine therapy
Anti-TNF-alpha for rheumatoid arthritis and anti-IL-17 for psoriasis (successful)
IRAP (IL-1 receptor antagonist protein), also termed ACS (autologous conditioned serum) foe equine osteoarthritis
Hygiene hypothesis (other names: old friends hypothesis or biome depletion theory)
The basis for helminth-based therapies in autoimmune diseases
Increase in allergic or autoimmune diseases due to reduced exposure to infections (debatable)
The hygiene hypothesis postulates that a reduction in the frequency of infections contributes directly to the increase in the frequency of autoimmune and allergic diseases
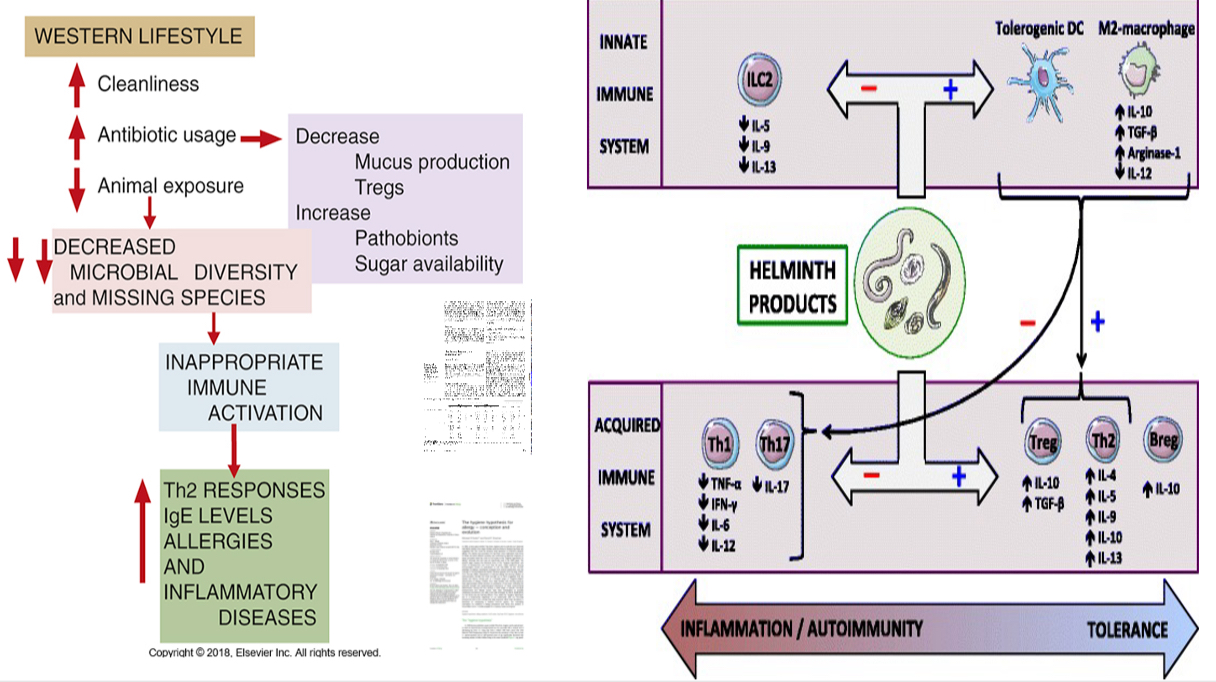
Immunoregulatory effects of helminths on the immune system
Helminthes exert their immunoregulatory actions by modulating cells of both the innate and adaptive immune system.
Regarding T-cells, helminthes may promote a Th2-type response and down-regulate Th1/Th17 differentiation, leading to increased Th2-type cytokine (IL-4, IL-5, IL-9, IL-10, IL-13) and decreased Th1/Th17-type cytokine (TNF-α, IFN-γ, IL-6, IL-12, IL-17) secretion.
Furthermore, worms’ products enhance Treg cell proliferation, the latter hampering Th1/Th2/Th17 polarization mainly through the secretion of IL-10 and TGF-β.
Helminthes also promote a regulatory phenotype of B-cells, DCs, and macrophages. Both tolerogenic DCs and regulatory M2-macrophages contribute to switching from a Th1/Th17 to a Th2/Treg profile.
Finally, these parasites may hamper the proliferation of ILC2, a subset of innate immune cells responsible for allergic responses. Thus, helminths create a tolerant environment, ensuring their own survival but also protecting the host from immune-mediated conditions by limiting excessive inflammatory and autoimmune phenomena.