IB Chemistry HL (copy)
1/227
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
228 Terms
Avogadro's number
6.02 * 10^23
Molar mass (M) units
g / mol
Kelvin unit of temperature
Celcius + 273
STP in data booklet is 0 degrees
Combined gas law, for changes to environment of a fixed mass of gas
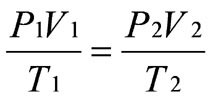
Ideal gas equation, for current conditions of a gas
PV=nRT
T in K
Equal volumes of different gases at the same temperature and pressure...
Have equal numbers of particles
Solution
homogeneous mixture of a liquid (solvent) and another substance (solute)
[Solute]
concentration of the solute, mol/dm3 or mol/L
Decimeter (unit)
mm < cm < dm < m
Isotope
Atom with different number of neutrons, differs in physical properties that depend on mass (ie. density)
Orbital
Region of space where 2 electrons (with opposite spin) can be found
Probable electron density
S orbital
sphere shape 1 per energy level
P orbital
dumbbell shape 3 per energy level
What order to fill orbitals in when drawing orbital diagrams
Aufbau principle — the triangle with diagonal lines, orbitals with lower energy are filled before those with higher energy
Hund's rule — Every orbital in a sub-level is singly occupied with electrons of the same spin before any one orbital is doubly occupied
D orbital
5 per energy level
F orbital
7 per energy level
Electron configuration notation: 1s^2
1 is energy level, s is orbital type, 2 is how many es in the sub-level
Remove electrons (for cation) from which orbital in electron configuration?
Highest level/shell number! Not Aufbau-diagonals order
First ionization energy definition
Energy required to remove one mole of electrons from one mole of gaseous atoms
Energy required to transition electron from first energy level/shell to ∞
Ionization energy trend
Increases to the top (less electron shielding) and right (increase in effective nuclear charge)
Successive ionization energies (first, second, etc)
Should increase progressively (since larger proton:electron ratio as you go)
A huge increase means you're trying to remove an electron from a noble gas electron configuration (aka a new energy level, not just new sub-level)
Elements in the same period have...
outer electrons in the same energy level
Transition metals (definition, the excluded elements)
must have an incomplete d sub-level in one or more of is oxidation states (aka not Zinc which is full, and not Scandium which is empty)
Common characteristics of transition metals
Variable oxidation number (ion charge), coloured compounds, magnetic properties, form complex ions (a metal ion bonded to a ligand via a covalent bond)
Effective nuclear charge on an electron
the nuclear pull experienced by the outer electrons in an atom, taking into account the shielding effect of inner full shells of electrons
Atomic radius trends
increases to the bottom (just bigger nucleus and more electron shells) left (less effective nuclear charge on valence electrons, they're further away)
Is anion/cation bigger or smaller than normal atom (in terms of atomic radius)
anion > atom since additional electron repulsion increases radius of outer shell
cation < atom since you lost the outer shell completely
Isoelectronic
Same electron configuration (ie. Na+ and Mg 2+)
Electron affinity (definition and trend)
the energy released when one mole of electrons is added to one mole of gaseous atoms
opposite trend to atomic radius (increases to the top right)
Electronegativity (definition and trend)
a measure of the attraction of a nucleus for bonding electrons
same trend as electron affinity, opposite trend as atomic radius — excluding noble gases
Melting point trends in metals
decrease down group 1, increase in ionic radii reduces the force of the attraction between the M+ ions and the delocalized electrons
Melting point trends in non-metals
increase down group 17, increase in the strength of London dispersion forces (because valence electrons held less tightly and form temporary dipoles more easily) with increasing number of electrons
Ligand
An ion or molecule with an electron pair to donate to form a covalent (coordinate) bond
Lewis base
Transition metal coloured complexes (why, when)
When ligands bond to the central metal ion, repulsion between the electrons in the ligands and those in the d orbitals of the transition metal ion causes the five d orbitals to split into two sets of different energy (non-degenerate orbitals)
electrons can transition from the lower set to the higher set of d orbitals by absorbing energy, which are known as d-d transitions — we see the complementary colour of the light wavelength absorbed
When do transition metals (coloured complexes) absorb shorter wavelengths of light
ligands higher in the spectrochemical series produce a larger splitting of the d orbitals = takes more energy for electrons to make d-d transitions = absorbs shorter wavelengths of light
Ionic compounds (definition, melting pt, solubility)
ions held together in crystal lattice structures (surrounded by ions of opposite charge) by ionic bonds, electrostatic attraction (mutual attraction between opposite charges)
formula expressed by simplest ratio
high melting point, soluble in polar solvents (water)
Are ionic compounds conductive
conduct electricity when molten or aqueous but NOT when solid
What differences in electronegativity define a bond as non-polar covalent, polar covalent, or ionic?
non-polar covalent = EN difference under 0.5 polar covalent = EN difference between 0.5 and 1.7 ionic = EN difference greater than 1.7
Covalent compounds (definition, what impacts bond length, what causes polarity, solubility)
form by the sharing of 2 electrons
Increasing number of bonds = shorter and stronger bonds
Polar bonds form when the two atoms bonded together have different electronegativity values (more than 0.5 difference) (exception: if molecular shape results in cancellation of polar bonds)
Like dissolves like — polar covalent soluble in polar solvent, non-polar covalent soluble in non-polar solvent
Coordinate covalent bond
when both shared electrons are from the same atom
Are covalent compounds conductive
Generally not good electrical conductors as solids
Conductive when aqueous IF they are able to ionize in solution (ie. HCl)
But most polar covalent molecules only dissolve by breaking apart IMFs, not internal bonds
Pure covalent bonds
non-polar covalent bonds, equal sharing of electrons
ex. HOBrFINCl
Dipoles
oppositely partially-charged poles, or regions, in a molecule
due to uneven sharing of bond electrons
Exceptions to octet rule
BeCl2 and BF3 (less than an octet)
and PCl5 and SF6 (more than an octet because central atom from third period or beyond)
Resonance structures
when there is more than one possible position for a double bond
a structure that occurs when it is possible to draw two or more valid electron dot structures that have the same number of electron pairs for a molecule or ion
The resulting electron structure (actual structure) of the molecule is given by the average of these resonance structures
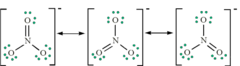
Giant covalent molecules
Atoms continuously covalently bonded
Number of atoms in "molecule" will vary with size of crystal
Exist in different allotropes (forms) (ie, diamond vs graphite)
Chemical formula represents the exact number of atoms in a molecule
Most common elements to form giant covalent molecules
C or Si
Characteristics of giant covalent molecules that are DIF from regular molecules
Exception to covalent compound traits: very hard, brittle, high melting point
Strength comes from 3D arrangement held together by covalent bonding
Allotrope
two or more different physical forms in which an element can exist (ex. carbon as graphite, charcoal, and diamond)
What is an IMF and which IMFs are stronger?
Overcome these when changing states, weaker than electrostatic attraction
Hydrogen bonding > dipole-dipole attraction > LDFs
Hydrogen bonding
Dipole-dipole attraction for polar molecules that have H bonded to FON
London dispersion forces
Dipole induced-dipole: a molecule with a dipole can induce a dipole in an otherwise neutral molecule
Instantaneous dipoles: due to instantaneous dipoles formed by random electron motion, weak and short lived
Van der waals forces
both dipole-dipole forces and LDFs (aka most IMFs minus sort of Hydrogen bonding)
Metallic lattice structures (definition, conductivity, physical characteristics)
Metal atoms are held together by the electrostatic attraction between a lattice of positive ions and delocalized electrons
Electrical and thermal conductivity (mobile electrons), malleable
Valence electrons move very quickly throughout the metallic crystal, attracted to the positive core of all positively charged metal ions
What impacts bond strength in metallic lattice structures
strength of metallic bond increases with charge on ion and decreases against the radius of the ion
Alloy
Non-directional bonding in mixed metal lattice structures
homogeneous mixtures of metals that cannot be separated by mechanical means
Lattice energy definition
The energy required to break 1 mole of ions in crystal lattice into infinitely spaced gaseous ions
The amount of energy in ionic bonds / the strength of the bonds
When is lattice energy greater
Greater when greater ion charge (greater electrostatic attraction)
Greater when smaller atomic radius (more effective nuclear charge)
Covalent bonds
different atoms' orbitals overlap and share electrons, results in new, differently shaped electron probability cloud with es attracted to positive nucleus of both atoms
Atoms and ions are stable when...
they are isoelectronic to a noble gas (form bonds to do that)
Bond order (for an atom with covalent bonds)
the number of bonds it has (ie, 1 for single a bond)
As bond order increases, the bond length decreases and bond strength increases
Can be a decimal for structures with resonance
Polyatomic ion
internal covalent bonds, external electrostatic attraction
Molecule with covalent bonds, formed with X extra es
Dipole
unequal charge distribution along a bond or whole molecule
Free radical
unpaired atom with an unpaired electron
Drawing Lewis structures (molecules)
Sum the valence electrons from all atoms
Write the symbols for the atoms to show which atoms are attached to which, and connect them with a single bond
Complete the octets of the surrounding atoms using lone pairs of electrons, remaining electrons go on the central atom
If central atom does not have an octet, move lone pair(s) from surrounding atoms to form additional bonds
Formal charge (for Lewis structures)
Formal charge: the difference between the number of valence electrons on the free atom and the number of valence electrons on the bonded atom (FC = V - N - B/2)
The structure with the lowest absolute value of formal charges is the most correct
Electron domain geometry (VSEPR)
The arrangement of electron domains surrounding the central atom of a molecule or ion
Molecular geometry (VSEPR)
The arrangement of the atoms in a molecule (The nonbonding electron pairs are not included)
Hybridization
Electrons from full orbitals are "moved" so that as many orbitals in that energy layer as possible have one electron -- so you can form more bonds (the single bond in any single, double, or triple)
Hybridized orbitals become degenerate
s2 p2 becomes s1 p3 becomes sp3 (with no space, and don't need to label the 1)
What order do you write sub-levels in when identifying electron configuration?
1s^2 2s^2 ... notation
In order of shell/level, not the order you fill them in
Ex: [Ar] 3d^10 4s^2
A common transition metal oxidation state
+2 because they lose their 4s shell
Except Sc because it loses 3d1 really easily
Acid-base properties of the period 3 oxides
change from basic to acidic across the period
State of the period 3 oxides
change from solid to gas across the period due to weakening IMFs
Alkaline meaning
basic
Drawing Lewis structures (ionic)
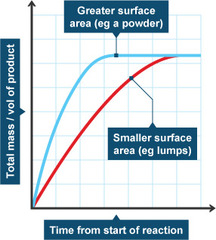
Time and Volume of product diagram, if you have a catalyst
They don't reach the same volume at the same time (don't intercept at the end)
degenerate orbitals
orbitals that have equal energy (ie. two P orbitals from the same shell, or hybridized SP orbitals from the same shell)
Pi bond
formed by the overlap of p orbitals on adjacent atoms, perpendicular to any sigma bond(s) between the same atoms
second bond in a double bond, formed without hybridization (or 2nd and 3rd in a triple bond)
delocalized electrons = greater stability
no free rotation

Sigma bond (σ bond)
Bond formed directly between two atoms by the head on overlap of orbitals
Single bond
Free rotation
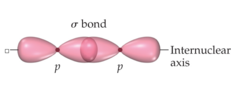
delocalized electrons
electrons that are free to move
electrons that are not associated with a single atom or a covalent bond
Alkane
C(n) H(2n+2)

Alkene
C(n) H(2n) One unit unsaturated (one unit is loss of 2H)
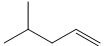
Ex: 4-methylpent-1-ene
Alkyne
C(n) H(2n-2)
Two units unsaturated (one unit is loss of 2H)
configurational isomers
can only be interchanged by breaking and reforming bonds
two types of configurational isomerism:
cis-trans or z-e
optical isomerism
cis/trans and Z/E isomerism (configurational isomers)
on either side of a non-rotating pi bond or cyclic molecule
if carbons on either side of the double bond have 1 identical substituent: cis (stuff on the same side) vs trans (stuff on opposite sides)
if carbons on either side of the double bond have no identical substituents: (Z) (halogen w highest atomic number or longest carbohydrate chain on zame zide) vs (E) (enemy sides)
Chiral molecule
contains a carbon atom (sometimes called a chiral carbon atom or a chiral center) that is bonded to four different atoms or groups
enantiomers
configurational isomers that are symmetrical but not superimposable
contains chiral carbons = a type of optical isomerism (which is a type of configurational isomerism)
Do enantiomers have the same properties?
identical chemical properties (except with other chiral molecules in the human body)
same physical properties, apart from their interaction with plane polarised light
Are enantiomers and diastereomers optically active?
an enantiomer rotates the plane of plane-polarised light
some diastereomers are optically active, some are not
Diastereomers
from configurational isomerism in molecules with more than one chiral carbon, if not all chiral carbons are symmetrical
not symmetrical and not superimposable
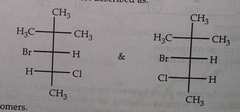
Do diastereomers have the same properties
can have different physical and chemical properties
Racemic mixture
optically inactive mixture made with equal amounts of two optically active enantiomers that cancel each other out
How many enantiomers does a molecule with n chiral centres have
2n optical isomers for an organic molecule with n chiral centres
structural isomers
have the same molecular formula but a different bonding arrangement among the atoms
structural isomers vs stereoisomers
structural = entirely different bonds just with the same chemical formula
stereo = same bonds, just different in spatial orientation
stereoisomers (configurational and conformational)
have identical molecular formulas and arrangements of atoms. They differ from each other only in the spatial orientation of groups in the molecule
conformational isomers can be interconverted by rotation round single covalent bonds, but configurational isomers cannot be interconverted without breaking a covalent bond
Configurational stereoisomers
cannot be interconverted without breaking a covalent bond
Conformational stereoisomers
can be interconverted by rotation round single covalent bonds
Alcohol (FG name, primary vs secondary vs tertiary)
hydroxyl functional group R-OH Ex: pentan-2-ol
tertiary is most stable in carbocations (in SN1 when the halogen is removed but the hydroxyl has not been added yet) because the electron cloud stabilizes the positive carbocation
primary has highest boiling point because OH more exposed for hydrogen bonding
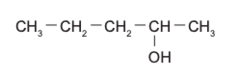
Does SN1 or SN2 make a racemic mixture?
SN1 if the 3 branches are all different lengths