Biology - Mendel & Pedigrees
1/225
Earn XP
Description and Tags
for the secod midterm or whatever brah
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
226 Terms
Autopolyploidy
Extra sets of chromosomes derived from the same species caused by accidents in mitosis or meiosis.
Colchicine
Binds tubulin.
Inhibits tubulin polymerization.
Disrupts spindle formation.
Used by plant breeders to induce polyploidy.
Also used as medicine (i.e. for gout).
Hybridization (Allopolyploidy)
Changes in chromosome numbers can create stable species in plants.
Wild cabbage (Brassica oleracea) has many cultivars, e.g. Broccoli and Cauliflower.
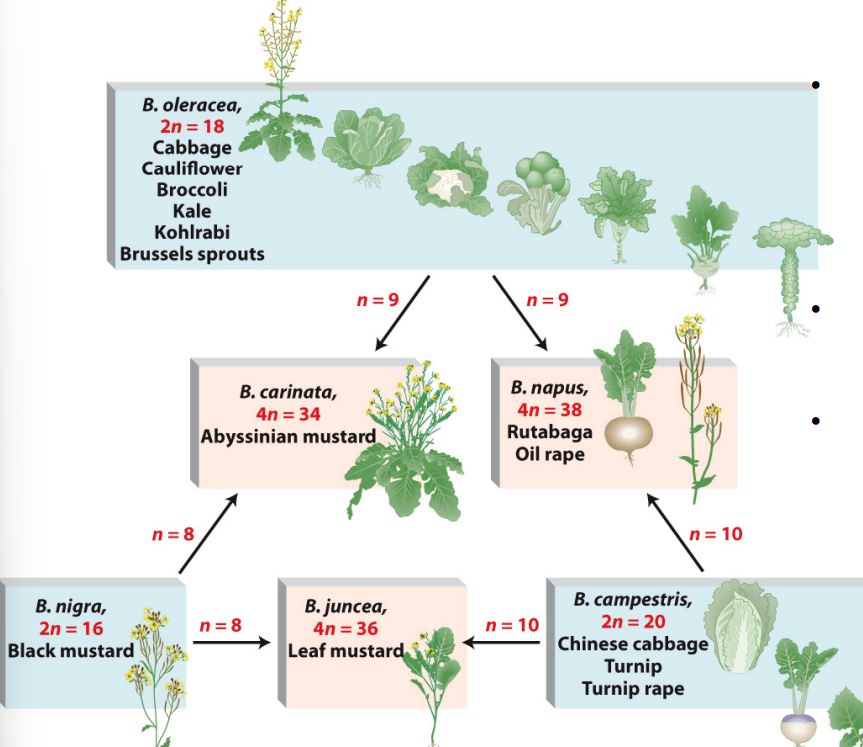
Allopolyploidy
A hybrid plant species that is derived from two or more species, containing two or more copies of each original genome.
Triploid Seedless Plants
Even-numbered polyploid plants are often bigger than their diploid counterparts.
Odd-numbered ploidy is often associated with sterility.
This is because homologous chromosomes cannot pair properly, and segregation would be uneven.
Without meiosis, seed production is aborted.
Propagating Sterile Triploids
To propagate such sterile plants:
Cuttings can be grown asexually (e.g. banana).
Tetraploid plant can be crossed to diploid plant to create triploid seeds (e.g. watermelon).
Cavendish Banana
Global industry worth $120 billion per year.
The Cavendish is triploid and sterile.
Trees are propagated asexually through cloning.
Genetic uniformity makes crops highly vulnerable.
Cavendish Crisis
Infected by Sigatoka complex (three ascomycete fungi).
Weekly fungicide sprays are required on most plantations.
Fungicide costs: ~$1,000 per hectare yearly (~35% of production).
Fungal resistance is increasing, threatening global extinction of Cavendish bananas.
Endoreplication & Endopolyploidy
Occurs when DNA replication repeats without mitosis or cytokinesis.
Results in polyploid tissues within an otherwise diploid organism.
Common in large, specialized cells with high synthetic activity.
Leads to increased gene expression capacity due to extra DNA copies.
Endoreplication & Endopolyploidy Examples
Human megakaryocytes: Have enlarged, lobulated nuclei; produce platelets (thrombocytes), which function in blood clotting.
Drosophila larval salivary glands: Contain polytene chromosomes (up to 1024 copies) forming giant chromosomes; synthesize glue to attach the pupa to solid surfaces.
Chromosomal Aberrations
Involve missing, extra, or rearranged chromosome regions.
Caused by two or more DNA strand breaks followed by incorrect repair.
Lead to abnormal chromosome structures that can disrupt gene function.
To survive cell division, the altered chromosome must retain a centromere and two telomeres.
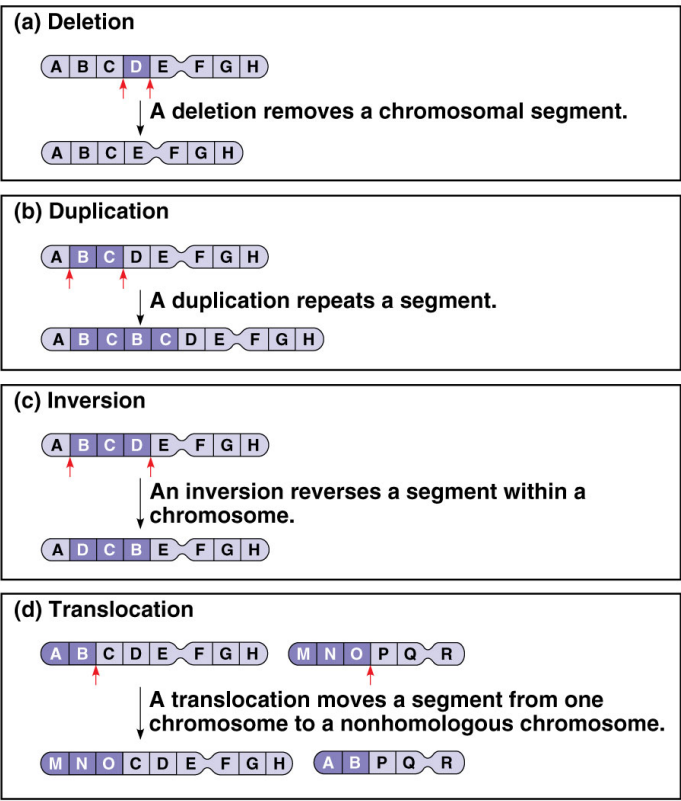
Deletions through Double-Strand Breaks
Two breaks occur within a single chromosome.
NHEJ (non-homologous end joining) proteins are recruited to repair all broken ends.
Repair may be imprecise, leading to loss of a DNA fragment.
Can result in chromosomal rearrangements or deletions.
Chromosome Deletions
Breaks occur in one or more regions of a chromosome.
A fragment lacking a centromere is typically lost and degraded.
Phenotypic severity depends on the size and function of deleted genes.
Deletions - Phenotypic Effects
Depend on the size and gene content of the deleted region.
Loss of essential genes disrupts development and cellular function.
Example: Cri du Chat syndrome — deletion on the short arm of chromosome 5.
Causes mental deficiencies, facial anomalies, and a catlike cry even with one abnormal chromosome copy.
Terminal Deletion
Loss of one end of chromosome (chromosomal deletion).
Interstitial Deletion
Loss of an internal segment (chromosomal deletion).
Deletions and Duplications from Incorrect Crossover
Caused by unequal crossing over between homologous chromosomes.
Misalignment often happens at repetitive sequences.
Produces one chromosome with a deletion and another with a duplication.
Duplications are usually less harmful, and small ones often show no effect.
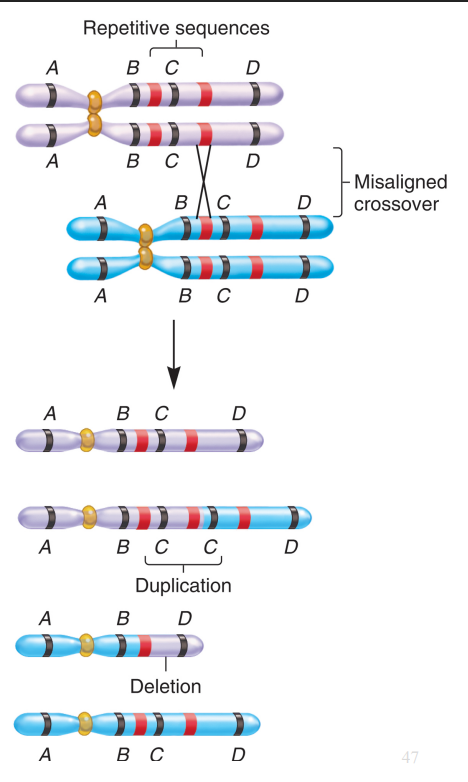
Tandem Duplication
Duplicated segment appears next to the original (e.g. AB●CDEFG → ABB●CDEFG).
Insertional (Displaced) Duplication
Duplicated segment is inserted elsewhere (e.g. AB●CDEFG → AB●CBDEFG).
Reverse Duplication
Duplicated segment is reversed in orientation (e.g. AB●CDEFG → AB●CDEFFEG).
Intrachromosomal vs Interchromosomal
Duplication on the same chromosome is intrachromosomal; on a different chromosome is interchromosomal.
Duplication and Evolution
Add new genes to chromosomes over evolutionary time.
Enable genetic innovation through extra gene copies.
Gene families arise from duplication and mutation of ancestral genes.
Allow one gene copy to maintain function while another diverges and gains new roles
Williams-Beuren Syndrome (WBS) Features
Small deletion (25–28 genes) on chromosome 7.
Broad forehead, short nose, full cheeks, wide mouth with full lips.
Narrow blood vessels → hypertension; short stature and mild to moderate intellectual disability.
Williams-Beuren Syndrome (WBS) Traits
Overfriendly and hypersocial, with a strong urge to approach strangers.
High sensitivity to certain sound frequencies (~50 Hz).
Remarkable musical affinity from an early age.
Warm, expressive personality despite cognitive challenges.
Genetic Basis of Hypersociability (WBS)
Dogs, but not wolves, carry mutations in WBS genes that promote hypersocial behavior.
These mutations parallel traits seen in Williams–Beuren syndrome in humans.
In mice, induced chromosome deletions in the same region cause hypersociability and other WBS-like features.
Reciprocal Translocations
Occur from double-strand breaks on two non-homologous chromosomes.
Involve a balanced exchange of chromosome segments.
During meiosis, they form quadrivalents, often yielding unbalanced gametes.
Non-Reciprocal Translocation
Represent a one-way transfer of DNA to a non-homologous chromosome.
Do not involve an equal exchange of material.
Are less common than reciprocal translocations.
Inversions
Occur when a chromosome segment reverses orientation.
Involve rearrangement of genetic material without gain or loss.
Usually benign, unless breakpoints disrupt vital genes.
Two types: pericentric & paracentric.
Pericentric Inversion
Centromere lies within the inverted region.
Involves both chromosome arms.
Can change the relative lengths of chromosome arms
Paracentric Inversion
Centromere lies outside the inverted region.
Involves only one chromosome arm.
May lead to abnormal chromatids during meiosis if crossing over occurs.
Inversions via Double Strand Breaks
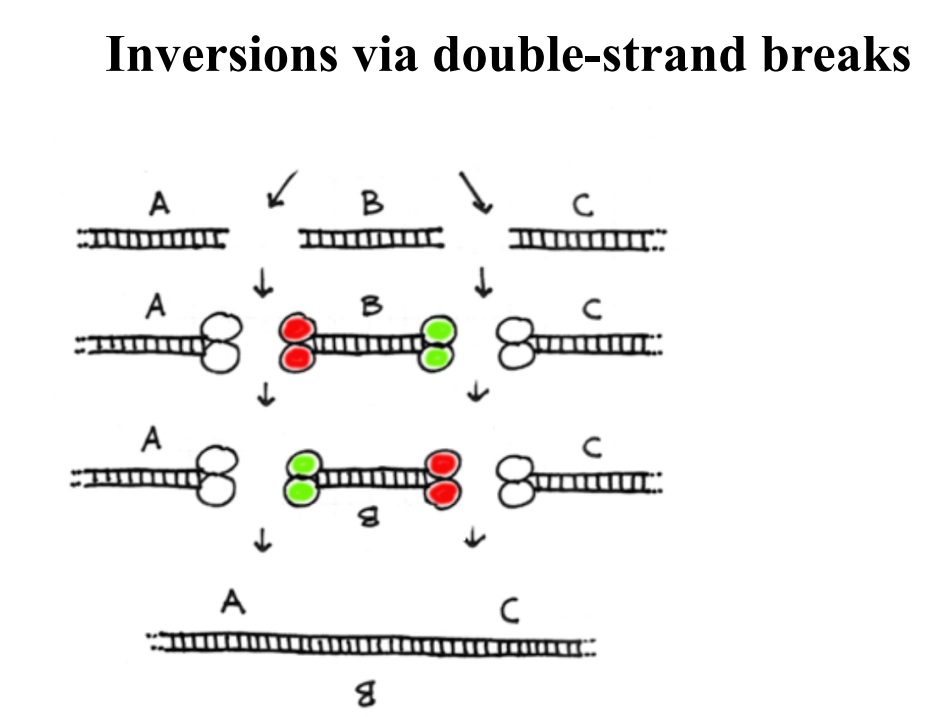
Nonhomologous End Joining
Accidental double strand break → loss of nucleotides due to degradation from ends → end joining → deletion of DNA sequence.
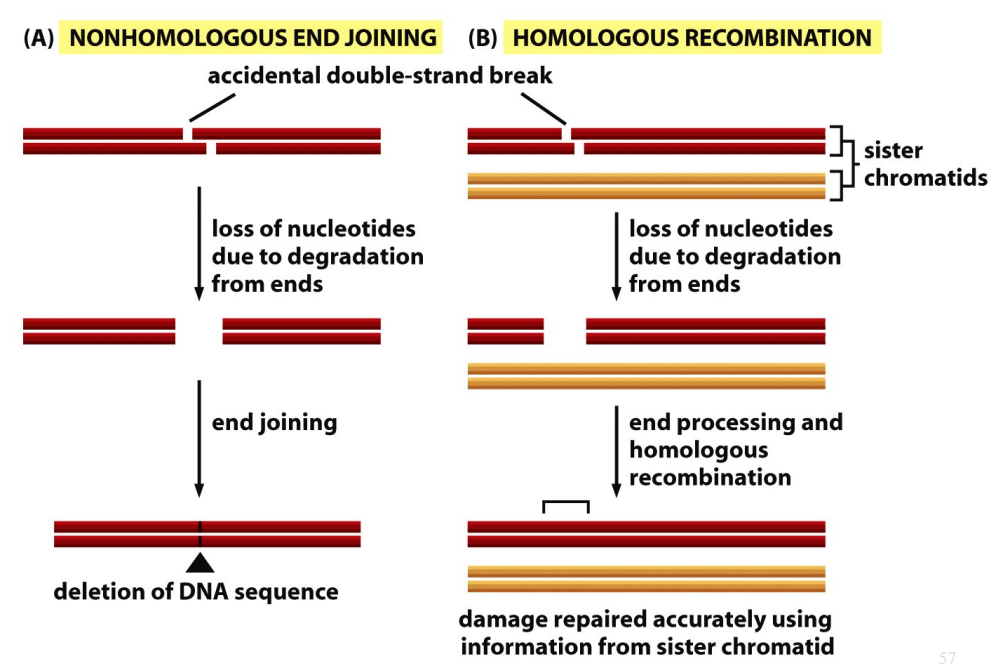
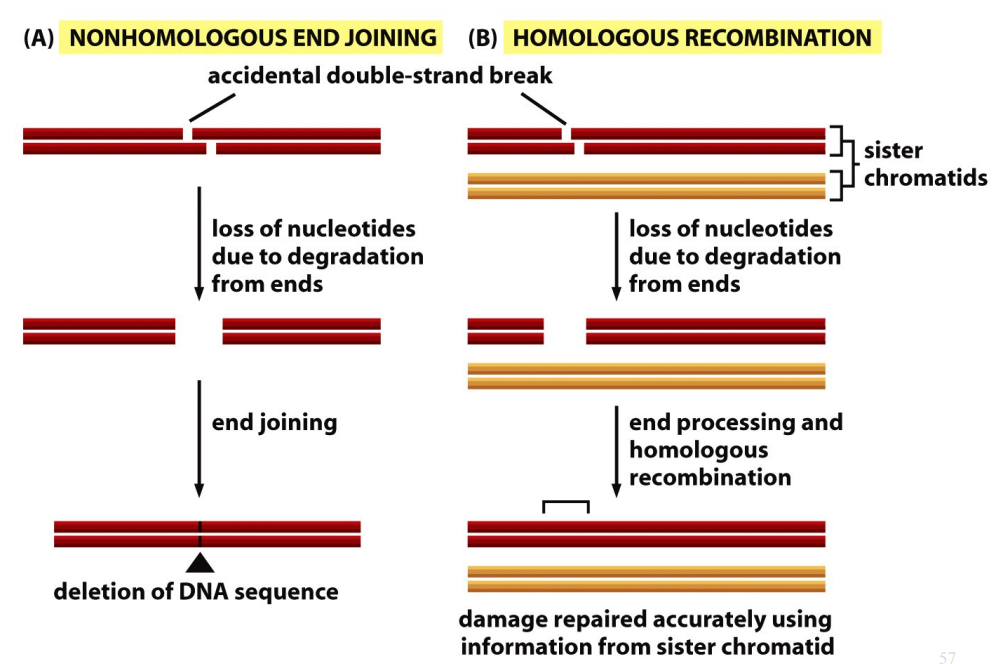
Homologous Recombination
Accidental double-strand break → loss of nucleotides due to degradation from ends → end processing and homologous recombination → damage repaired accurately using information from sister chromatid.
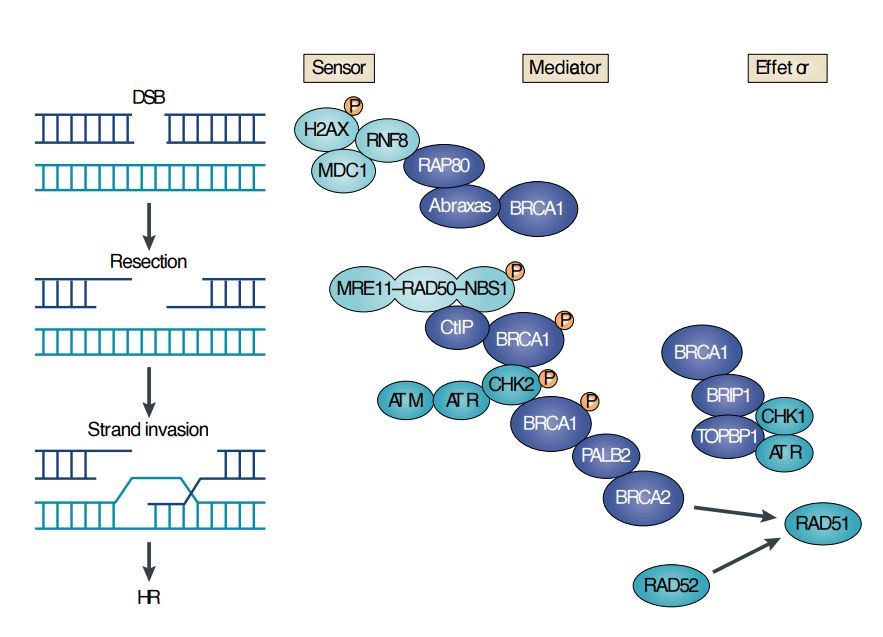
Non-Homologous End-Joining (NHEJ)
Repairs double-strand breaks in chromosomes.
Ku proteins, conserved from bacteria to humans, bind to the breaks.
Reattaches DNA fibers, often altering the original sequence.
Removes damaged nucleotides during repair.
Predominantly occurs in G1, before DNA replication.
Homologous Recombination (HR)
Repairs DNA by using the sister chromatid as a template, restoring the original sequence.
Occurs when a template is available, typically after replication (S phase) but before cell division.
Effective when daughter strands are still near each other post-replication.
Used in molecular genetics to introduce specific changes via a supplied “fake” template.
Proteins for HR
Homologous Recombination requires BRCA1 and BRCA2 proteins.
Angelina Jolie
All humans have two copies of BRCA1 per cell.
Mutations in BRCA1 increase cancer risk, often affecting only one allele in patients.
Double mastectomy performed in 2013.
Ovaries and fallopian tubes removed in 2015.
Alleles
Different forms of a gene that exist at a locus.
Wild type allele is typically the most commonly found allele in a population.
Variant/Mutant Allele
Different from wildtype allele.
May or may not adversely affect gene product.
May or may not result in detectable phenotype.
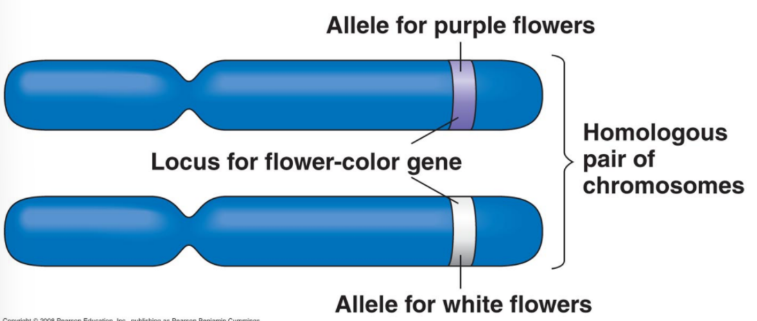
Homozygous
If identical alleles are present on both homologous chromosomes, the organism/cell is said to be _________ for that allele.
Two wild type alleles on two mutant alleles highlight the same allele, so _____ can be used for both.
FC/FC and fc/fc.
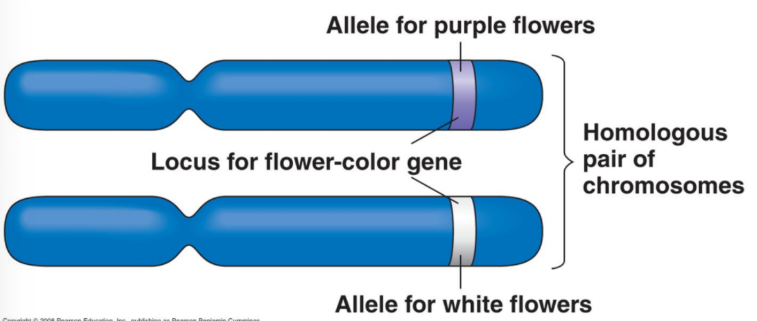
Heterozygous
If one allele is wild type and the other allele is not (i.e., a mutant allele), the organism/cell is said to be ________ for that allele.
FC/fc.
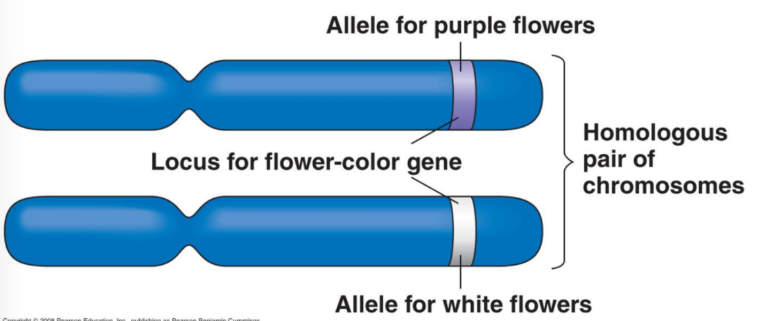
Allelic Series
The known mutant alleles for a given gene plus its wild-type allele.
All alleles of the same gene.
Wild-Type
Most common allele in a population and functionally normal.
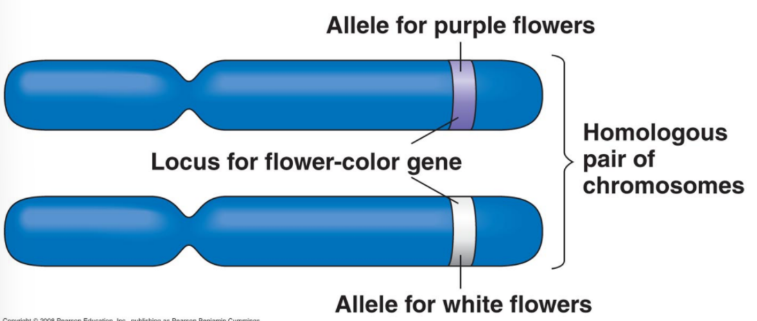
Flower Colour (FC) Gene
Purple flowers: functional protein produced.
White flowers: no functional protein (absence of pigment).
Gene named FC for “Flower Color.”
Functional allele = FC; non-functional allele = fc (both listed when referred to).
Alleles on Structural & Functional Levels
Different alleles can arise from mutations in various regions of a gene (e.g., promoter, coding region, or intron).
Structural changes in DNA (mutations) can affect whether a functional protein is produced.
Null Mutations
No functional protein produced; gene “OFF.”
m1, m2, m3, and m6.
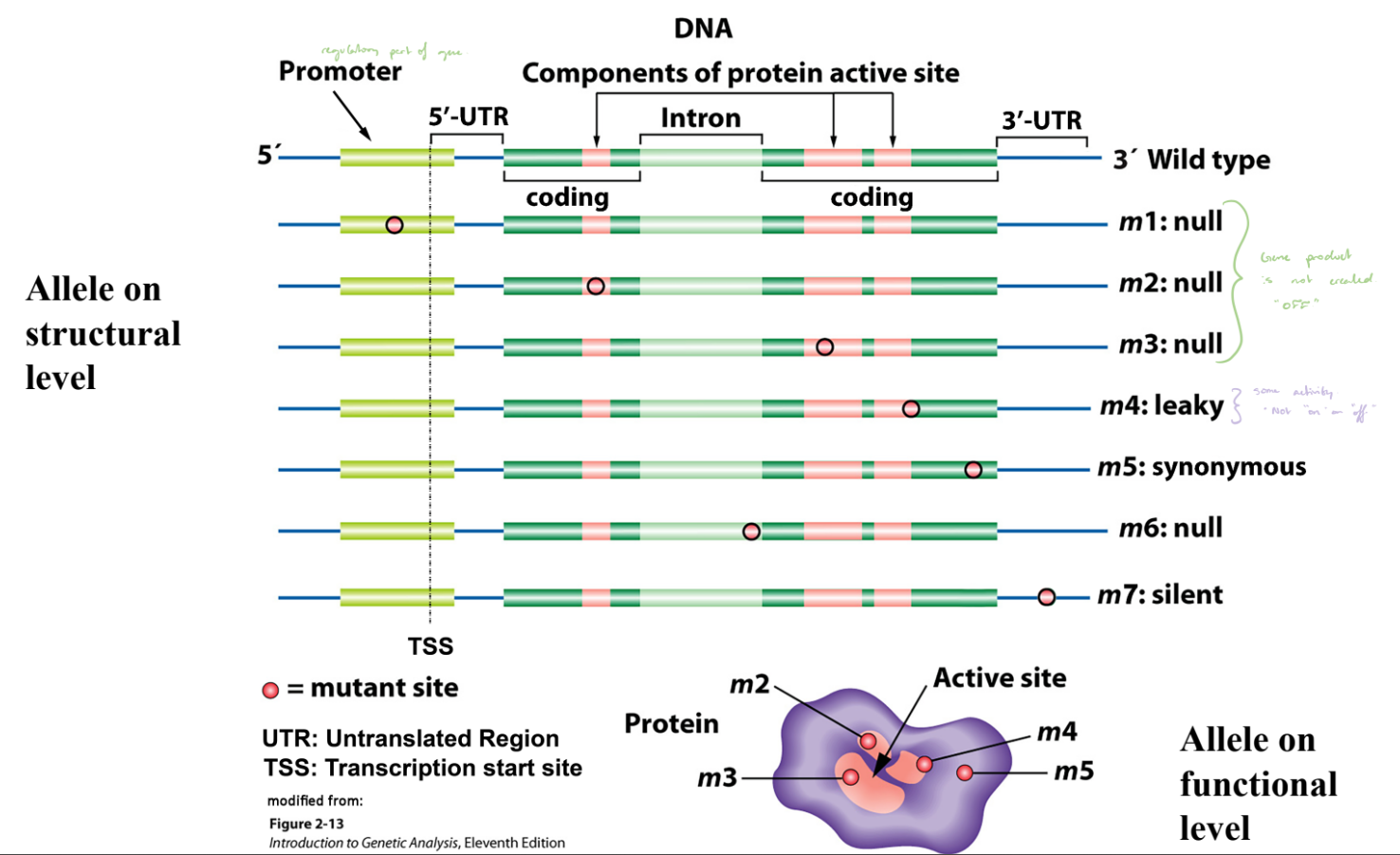
Leaky Mutation
Produces reduced protein activity; gene partially “ON.”
m4.
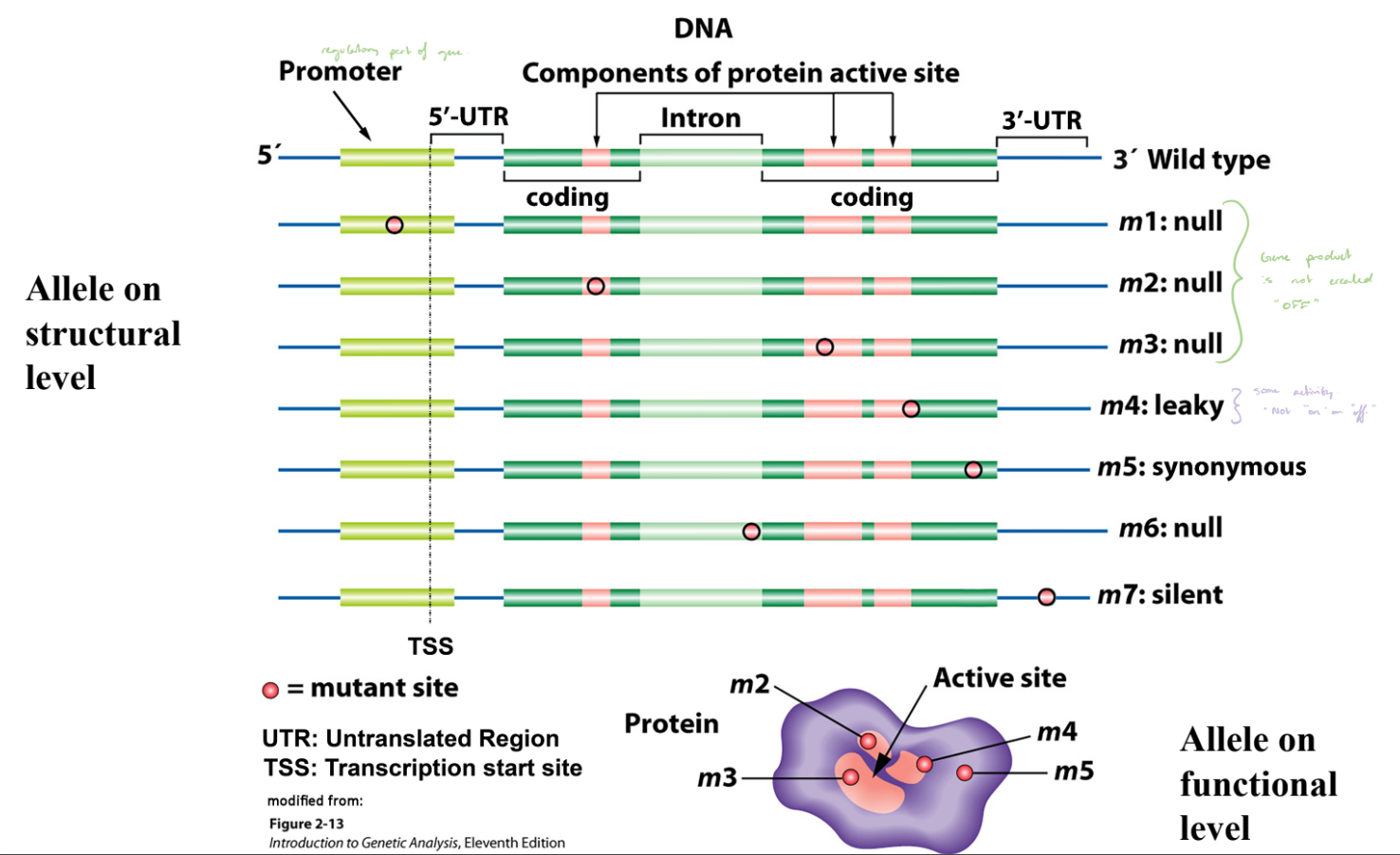
Synonymous Mutation
DNA change does not alter amino acid; no effect on protein function.
m5.
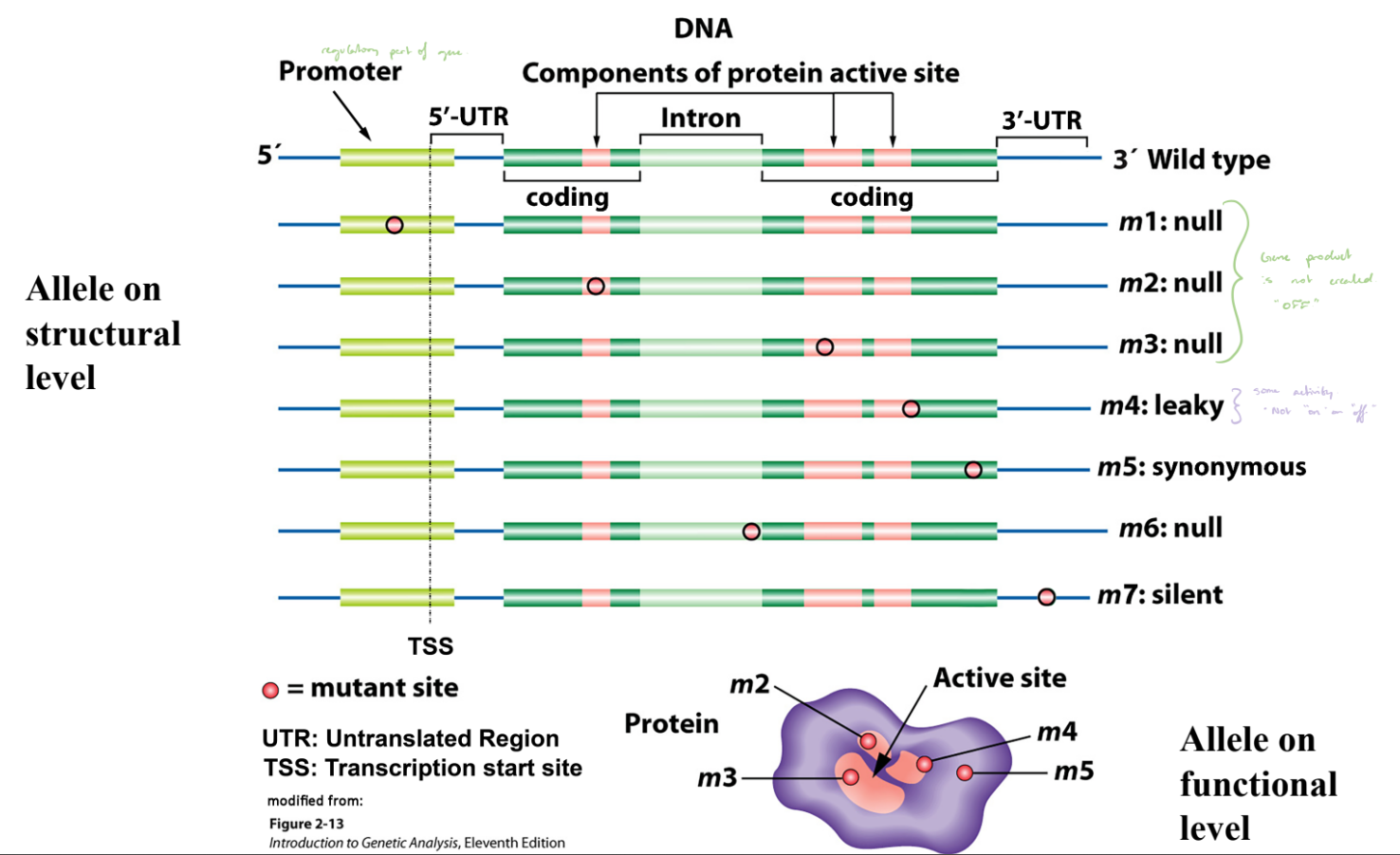
Silent Mutation
Mutation with no functional consequence.
m7.
FC / FC and fc / fc
Example of homozygous wild-type and mutant genotypes.
+/+ and -/-
General symbols for wild-type and mutant alleles.
FC⁺ / FC⁺ and fc⁻ / fc⁻: “+” and “−”
Denote wild-type and mutant forms of a specific gene.
“-” would suggest a complete loss of function.
FC⁺ / FC⁺ and fc¹ / fc¹
Superscripts identify specific alleles.
Has partial function and is specified in the scientific paper.
Uppercase/Lowercase Allele
Uppercase indicates a dominant allele; lowercase indicates a recessive allele.
Heteroallelic
Two different mutant alleles.
Also called transheterozygous or compound heterozygotes.
Hemizygous
A situation where a cell/organism has only one copy of a gene/locus/chromosomal region.
Hemizygous
A condition where a cell or organism has only one copy of a gene, locus, or chromosomal region.
Example 1: Deletion — the corresponding gene or region is missing on the homologous chromosome.
Example 2: Naturally single-copy genes — common for genes on X or Y chromosomes in XY individuals.
True-Breeding (Flower Ex)
When plants consistently produce offspring with the same trait when self-crossed (e.g., white × white → white).
Occurs when individuals are homozygous for the allele(s) controlling the trait.
Genetic Basis of Traits
May depend on one gene (FC) or multiple genes (FC, W, GD, HE).
Monogenic traits.
Polygenic traits.
Monogenic Traits
Controlled by a single gene (often with multiple alleles).
Polygenic Traits
Controlled by multiple genes, producing more complex variation.
Dominant Allele
In a heterozygous organism, its phenotype is expressed even when a recessive allele is present.
Typically represented by uppercase letters (e.g., A).
Dominance is relative — it may be observed at the organismal level but not always at the molecular or cellular level.
Recessive Allele
Its phenotype is masked by the dominant allele in a heterozygote.
Example: If A is dominant, a is the recessive allele.
Typically represented by lowercase letters (e.g., a).
The terms dominant and recessive are always defined in relation to each other.
Complete Dominance
The dominant allele (FC) completely masks the recessive allele (fc) in heterozygotes.
Both FC/FC and FC/fc produce the same phenotype (purple petals).
The fc allele is fully recessive when paired with FC.
Wild-type alleles are often dominant over their mutant counterparts.
Haplosufficient
A single functional copy of a gene is enough to produce the wild-type phenotype.
Deals with a molecular mechanistic view.
In FC/fc plants, one working FC allele provides sufficient gene product for normal color (purple petals).
Incomplete Dominance
Not all alleles show simple dominant/recessive relationships.
The heterozygote displays an intermediate or blended phenotype between the two homozygotes.
One functional copy is NOT sufficient for a wild type phenotype.
Four-o’clock Plants
Cᴿᴱᴰ / Cᴿᴱᴰ: Red petals.
Cᵂᴴᴵᵀᴱ / Cᵂᴴᴵᵀᴱ: White petals.
Cᴿᴱᴰ / Cᵂᴴᴵᵀᴱ: Pink petals (intermediate phenotype).
Heterozygotes show a phenotype midway between both homozygous parents.
Haploinsufficiency
One functional copy is NOT sufficient for a wild type phenotype.
One wild type copy is not sufficient to produce a wild type phenotype. Could result in complete or incomplete dominance.
More phenotypic difference.
Codominance I
Both alleles are fully expressed in the heterozygote, showing traits of both without blending.
Hbᴬ / Hbᴬ: Normal red blood cells.
Hbˢ / Hbˢ: Severe anemia, sickled cells.
Hbᴬ / Hbˢ: Some sickling under low oxygen, generally no anemia.
Sickle Cell Anemia
Caused by a point mutation in hemoglobin.
Missense mutation: A → T at position 6 of the beta-globin chain.
Too inflexible and cannot go through capillaries/BVs.
Hemoglobin A
Tetramer.
Two different genes that encode β-chain and α-chain.
Hbs
Hbˢ differs from Hbᴬ by a missense mutation substituting Valine for Glutamic Acid at position 6 of the β-chain.
Hbᴬ / Hbˢ: No anemia; some sickling under low oxygen.
Common in parts of Africa; one Hbˢ allele gives malaria resistance.
This selective benefit is a heterozygous advantage.
Codominance (II)
Genotypes & Phenotypes: Hbᴬ / Hbᴬ = normal RBCs; Hbˢ / Hbˢ = severe anemia, sickled RBCs; Hbᴬ / Hbˢ = no anemia, some slightly sickled RBCs.
Cause: Sickle Cell Anemia — point mutation in hemoglobin (Hbˢ vs Hbᴬ).
Dominance: Organism level — Hbᴬ is dominant; cellular level — incomplete dominance (some sickled cells).
Protein level: Both Hbᴬ and Hbˢ produced equally → codominance.
Dominant Negative Effect
The gene product from the mutant allele interferes with the gene product from the wild type allele (see also Muller’s morph “Antimorph”), blocking the wild type function.
Gain-of-Function Effect
The mutant allele acquires a new property not present in the wild type allele, and this new property causes a phenotype (see also Muller’s morph “Hypermorph” and “Neomorph”).
Dominance of a Mutant Allele Ex. 1
When a mutant protein interacts with the normal protein it can render the protein as non-functional.
The shape is altered.
Mutant affects the function by b/e non-functional or reduce its function.
This can be dimer, tetrameter, etc.
Dominance of a Mutant Allele Ex. 2
The normal protein is affected by binding to the mutated protein.
As a result, the dimer is non-functional.
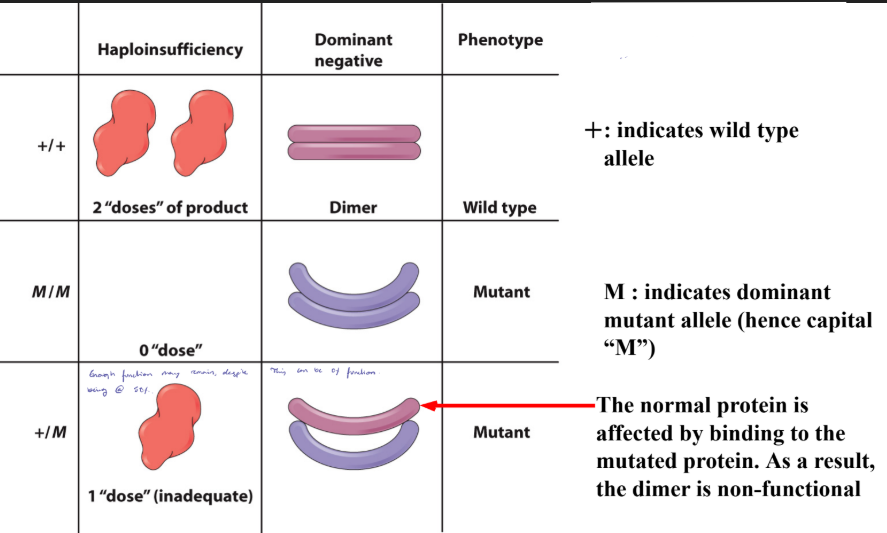
Mutant Allele Classification
Dominance/recessive describes relationships between alleles, not individual alleles themselves.
A more useful system classifies alleles based on the type of genetic defect.
H.J. Muller (Nobel Prize, 1946) designated five classes of mutant alleles called “morphs” (meaning “form”).
These classifications are still used today, sometimes alongside modern equivalents.
Amorph (Null) Mutations
“A” = absence; complete loss-of-function; also called null mutation.
Caused by deletions, missense mutations that inactivate protein, or nonsense mutations.
Classification is based on phenotype, not the type of molecular change.
Any mutation causing total loss-of-function is an amorphic allele.
Hypomorphs
“Hypo” = reduced; allele is partially functional but below wild-type levels.
Most commonly used term in Muller’s system; modern equivalents: partial loss-of-function or leaky.
Mutation may affect a regulatory element (reducing gene expression).
Or a point mutation may reduce the activity of the gene product.
Hypomorphs
Mutation in the regulatory region.
mRNA stability, doesn’t interact as good with a protein partner, etc.
All can lead to hypomorphism.
Porphyrias
Porphyrias affect heme biosynthesis (8 genes).
Most disease-causing alleles are amorphic or hypomorphic; patients usually heterozygous or heteroallelic.
Heme precursors build up in the mitochondria.
Complete loss of heme is lethal.
Porphyrias - Symptoms & Treatment
Light sensitivity, gum recession, extreme neuropathic pain.
Sensitivity in UV.
Heme or glucose injections can reduce symptoms.
Hypermorph (Increased Function)
“Hyper” = increased; gene is more active than wild type.
Modern equivalent: gain-of-function (but distinct from neomorphs).
Cause: regulatory mutations increasing expression or coding mutations making protein more active; normal product, just more or more active.
Gene product is produced in normal cellular context (unlike neomorphs).
Antimorphs
“Anti” = against; mutant gene product antagonizes wild-type function (in a heterozygous setting).
May dimerize with wild-type protein and inactivate it.
Can also compete for substrate or binding partner.
Usually dominant in inheritance.
Myostatin Mutations
Function: Myostatin represses muscle growth; loss-of-function increases muscle mass.
Cattle: Stop mutation → amorph (no myostatin).
Sheep: Point mutation in non-coding mRNA creates microRNA binding site → hypomorph.
Outcome: Reduced myostatin activity increases muscle; mutation type determines amorph vs hypomorph.
Neomorphs
“Neo” as in “new.”
The mutant gene product acquires a new function not found in the wild type protein.
This could involve new interactions, new substrates, or expression in the wrong cell type or developmental stage.
Are generally dominant or semidominant.
Oncogenes - Relocation
Example: Burkitt lymphoma caused by relocation of MYC oncogene next to a new regulatory element.
MYC: normally important for cell proliferation.
Mechanism: Recombination with chromosome 14 places MYC under incorrect regulatory control.
Result: gain of new function leading to abnormal cell proliferation (neomorph).
Oncogenes - Hybrid
Disease: Chronic Myelogenous Leukemia (CML).
Mechanism: Translocation between chromosome 9 (ABL) and chromosome 22 (BCR1).
Result: Formation of BCR-ABL fusion gene → hybrid oncogene.
Proto-Oncogene
The wild-type version of the oncogene.
Mutation Classification
Classification schemes are not mutually exclusive; they describe mutations at different levels:
DNA/chemical level: e.g., frameshift.
Gene product level: e.g., functional vs nonfunctional.
Phenotypic level: mutant vs wild type.
Allelic Interactions
Dominant vs recessive, incomplete (semi-) dominance, codominance.
Patterns depend on perspective, e.g., cell vs organism.
Muller’s Morphs
Classify all types of mutations.
Amorph, hypomorph, hypermorph, neomorph, and antimorph.
Complementation Tests
Used to determine if mutations are in the same or different genes.
Mutations that complement: occur in different genes → non-allelic mutations.
Mutations that fail to complement: occur in the same gene → allelic mutations.
Complementation in Genetic Screens
Complementation tests help identify new mutants.
After a genetic screen, we may have multiple mutants but don’t know if the same gene is affected more than once.
Complementation allows us to group mutants by gene.
Understanding this requires knowing how genetic screens are conducted.
Chemical Mutagenesis (Classic Approach)
Method: Feed a chemical mutagen, usually to males, to mutagenize sperm.
Efficiency: More efficient than X-rays; one male can fertilize many females.
Common mutagen: EMS (ethylmethane sulfonate).
Effect: Mostly G→A and T→C transitions; used in Drosophila, C. elegans, Arabidopsis.
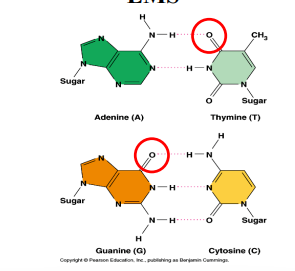
EMS-Induced Mutations
EMS (ethylmethane sulfonate, C₃H₈SO₃) is an alkylating agent that adds ethyl groups to nucleotides.
Causes mispairing during DNA replication → point mutations.
Commonly used in genetic screens.
Efficient for creating G→A and T→C transitions.
Mutant Hunts = Genetic Screens
Animals are mutagenized and thousands of progeny are examined to isolate new mutants.
Heidelberg Screen (1979–1980, Drosophila): analyzed abnormal larval cuticle patterns.
Isolated 600 mutants representing 120 genes using complementation tests.
One of the most famous mutant hunts in developmental biology.
Heidelberg Screen Impact
Revolutionized understanding of developmental processes.
Many genes discovered are conserved in humans.
Genes serve as models for human diseases.
Nobel Prize 1995 awarded to Eric Wieschaus & Christiane Nüsslein-Volhard.
Preaxial Polydactyly
Can be caused by mutation in the regulatory region of the human sonic hedgehog gene, which is important for limb development.