Chapter 7- Covalent and Metallic Bonding
When non-metals react with one another, they share electrons instead of transferring them just as in the diatomic elements.
The bond formed by sharing of electrons between non-metals is the covalent bond.
Sharing of one pair of electrons, i.e. two electrons, forms one covalent bond. Hydrogen has one covalent bond while nitrogen has three.
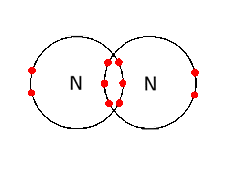
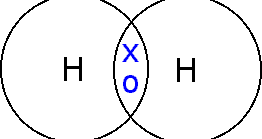
One covalent bond is represented by one line in the structural formula.
Compounds linked by covalent bonds are called covalent compounds or molecular compounds. Water, carbon dioxide, and methane are examples.
Within each molecule of a simple molecular compound, there are weak intermolecular forces of attraction so they have low densities, and melting and boiling points.
Most simple molecular compounds are soluble in organic solvents but not in water.
They do not conduct electricity in any state due to lack of mobile electrons/ions.
GIANT MOLECULAR STRUCTURES
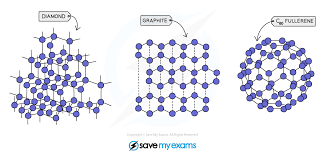
Diamond and graphite, allotropes of carbon have giant covalent structures.
Giant molecular structures are usually solids at r.t.p, have high melting and boiling points, and high densities, and do not conduct electricity in any state (except graphite).
Graphite is slippery so it is used as a lubricant while diamond is hard and shiny so it is used for cutting purposes.
Sand (silicon dioxide) is another giant molecular structure.
Common prefixes to name covalent compounds are mono, di, tri, tetra, penta and hexa.
METALLIC BONDING
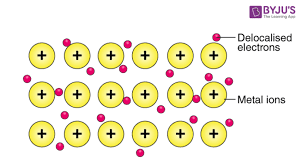
In metals, electrons break free of the atoms forming cations and become delocalised themselves. The electrons and cations attract each other due to opposite charges on them.
The force of attraction between positive metal ions and ‘sea’ of delocalised electrons is the metallic bond.
Since there are a lot of free electrons that move throughout the metallic structure, metals are good conductors of heat and electricity.
Metals are ductile and malleable because when force is applied, layers of atoms can slide over one another without disrupting the structure.
Due to strong forces of attraction between electrons and ions, metals have high melting and boiling points, and densities (exception: Group I)