Chapter 16 Topics
1/36
Earn XP
Description and Tags
Enthalpy And Spontaneity | Entropy | 2nd and 3rd Laws of Thermodynamics |
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
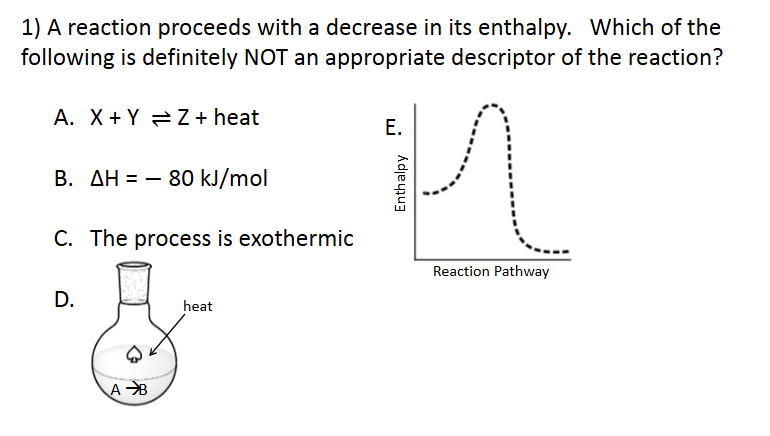
Enthalpy and Spontaneity
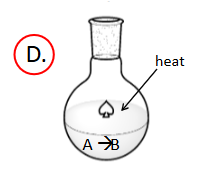

Enthalpy and Spontaneity
E. All of the above

Enthalpy and Spontaneity
A. The surroundings absorb heat
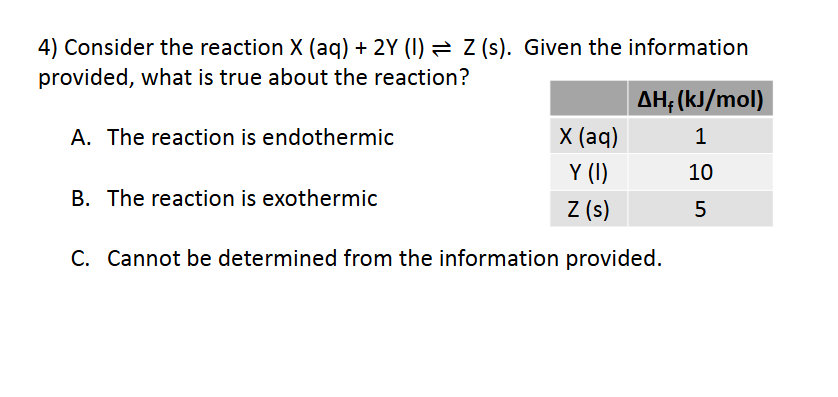
Enthalpy and Spontaneity
B. The reaction is exothermic

Enthalpy and Spontaneity
E. -16 kJ/mol

Enthalpy and Spontaneity
B. ΔH = +100 kJ/mol
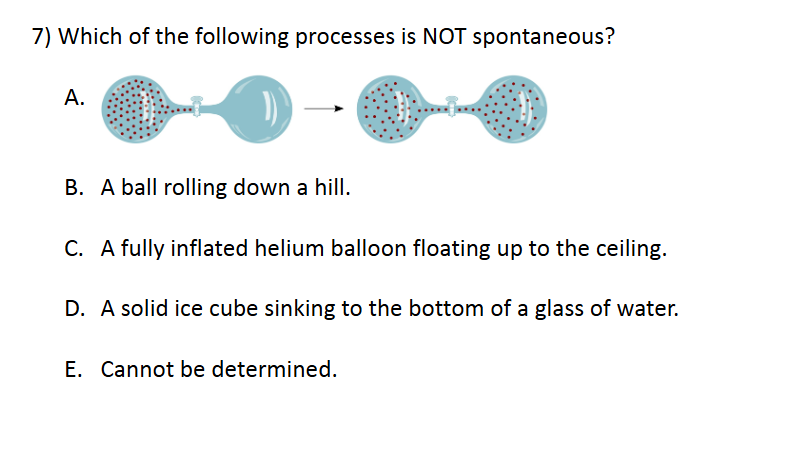
Enthalpy and Spontaneity
D. A solid ice cube sinking to the bottom of a glass of water
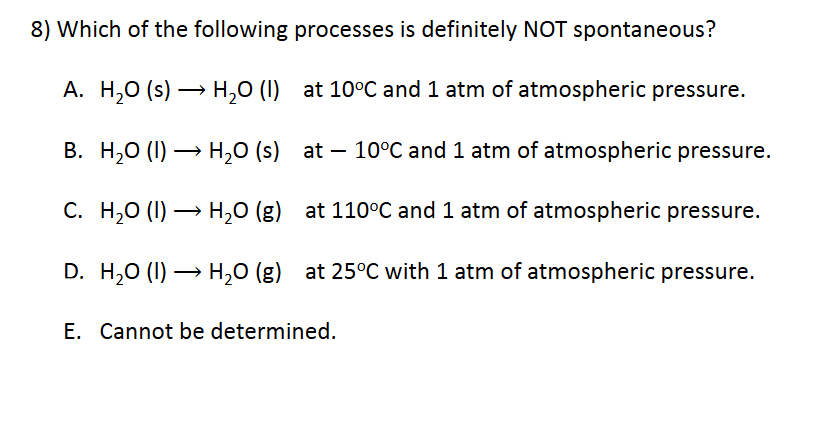
Enthalpy and Spontaneity
D. H2O (l) ⟶ H2O (g) at 25o C with 1 atm of atmospheric pressure

Enthalpy and Spontaneity
E. Cannot be determined

Enthalpy and Spontaneity
E. Cannot be determined
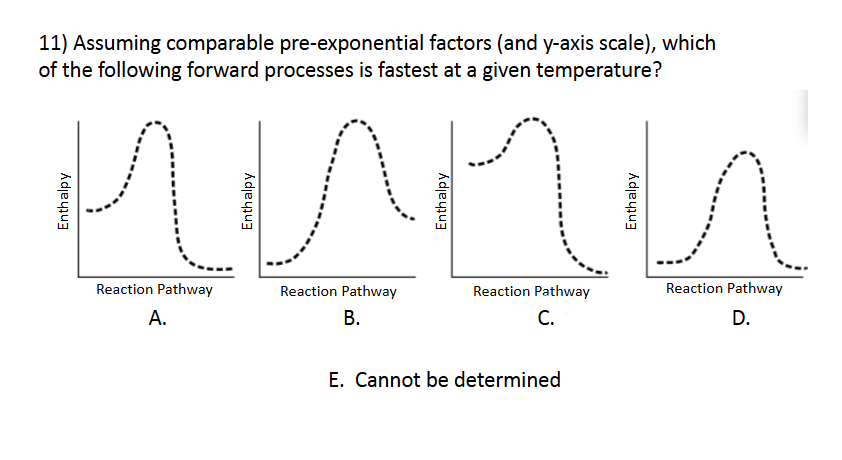
Enthalpy and Spontaneity
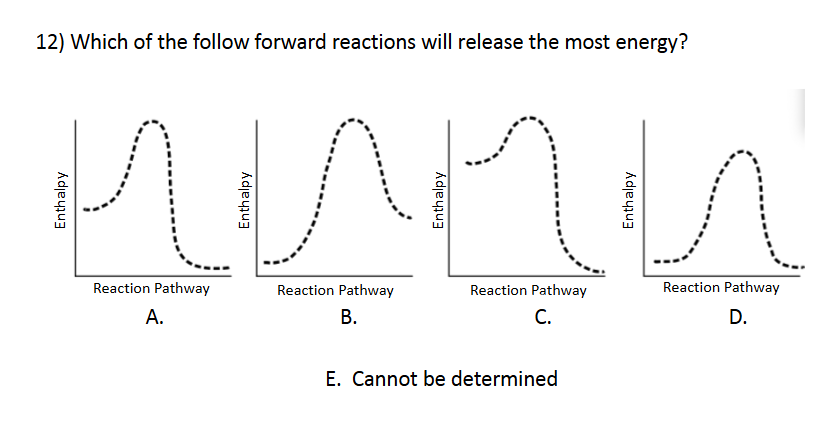
Enthalpy and Spontaneity
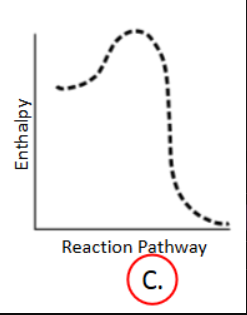
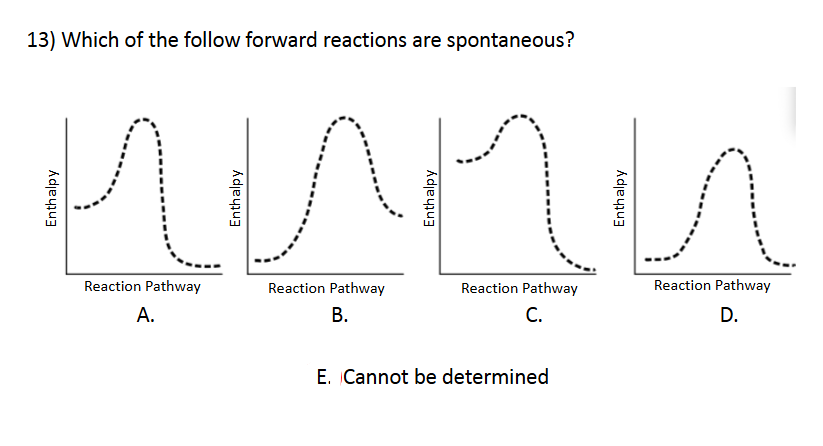
Enthalpy and Spontaneity
E. Cannot be determined
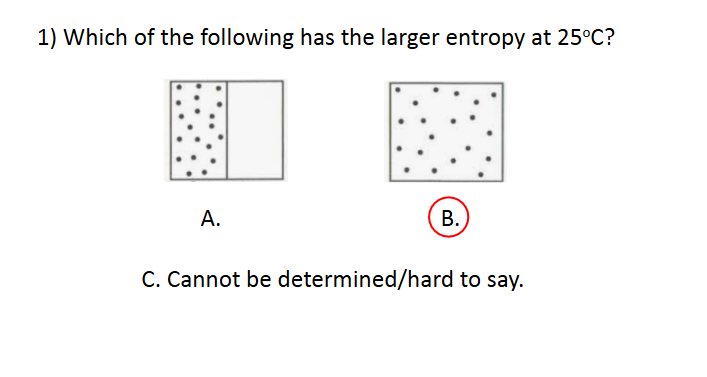
Entropy
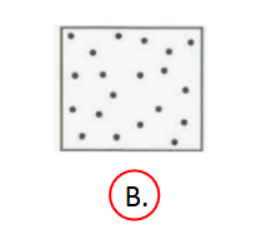
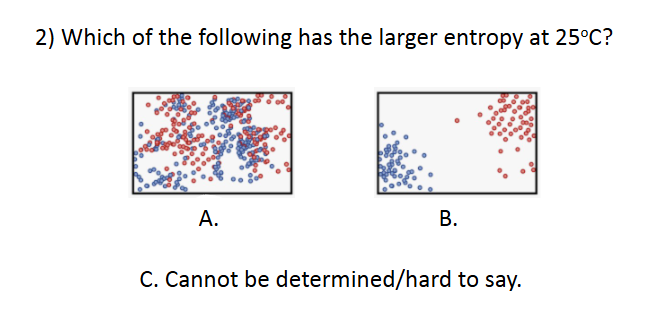
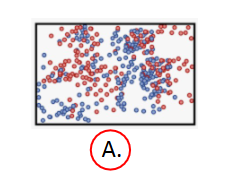
Entropy
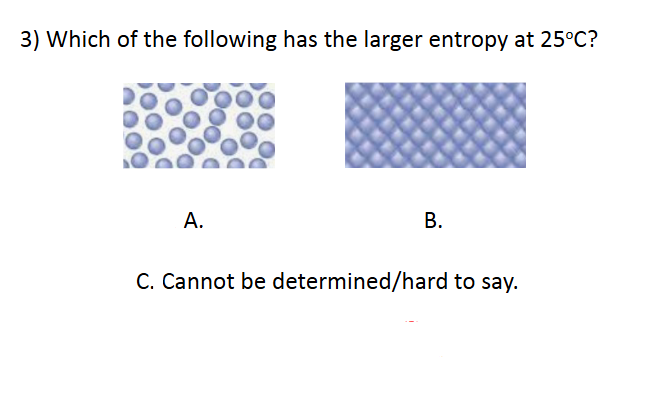
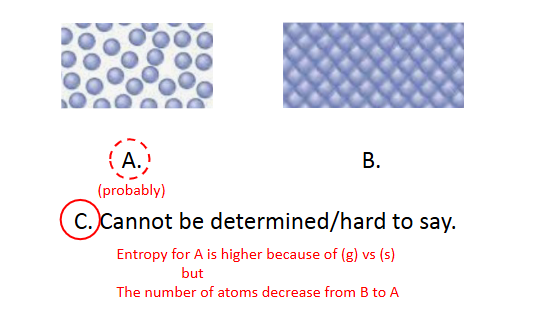
Entropy

Entropy
C. H2O (g)

Entropy
B. CH3OH (l) at 80o C
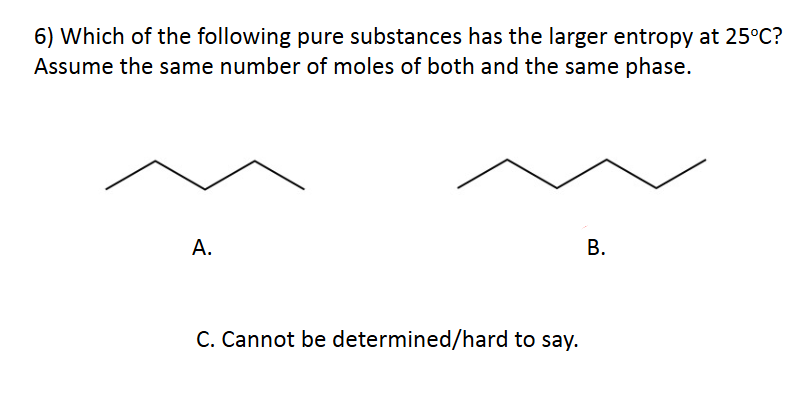
Entropy
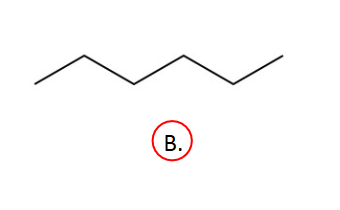

Entropy
D. Cannot be determined/hard to say

Entropy
C. A (s) at -20o C

Entropy
A. The entropy of the system (reaction) increases
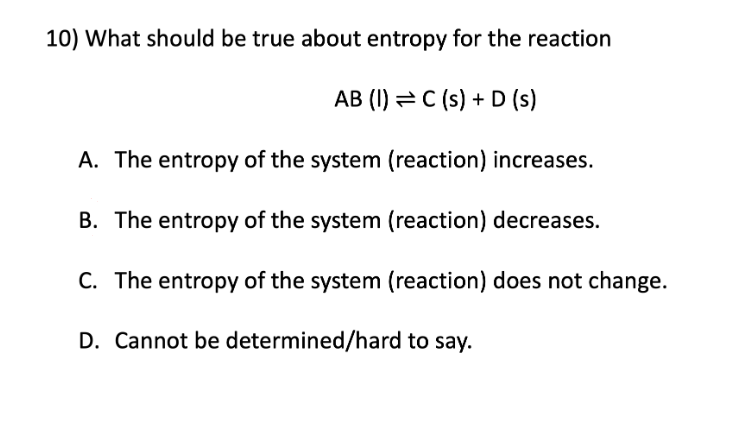
Entropy

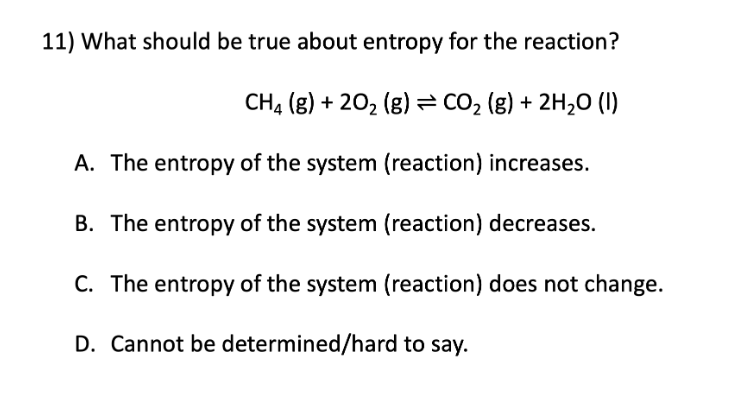
Entropy
B. The entropy of the system (reaction) decreases
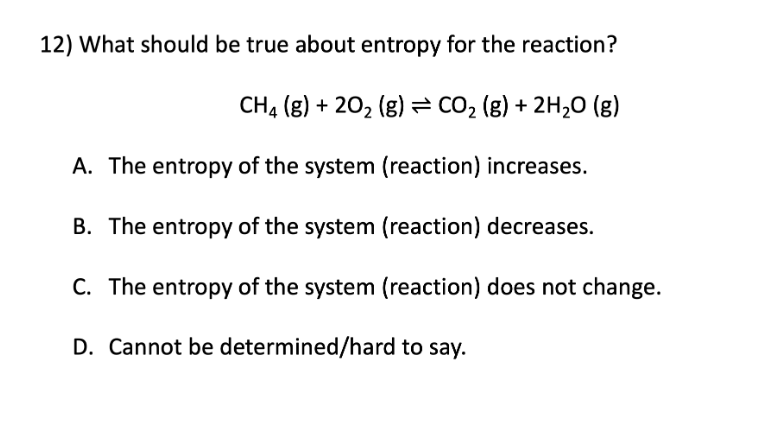
Entropy
D. Cannot be determined/hard to say

2nd and 3rd Laws of Thermodynamics

2nd and 3rd Laws of Thermodynamics

2nd and 3rd Laws of Thermodynamics

2nd and 3rd Laws of Thermodynamics

2nd and 3rd Laws of Thermodynamics

2nd and 3rd Laws of Thermodynamics
D.
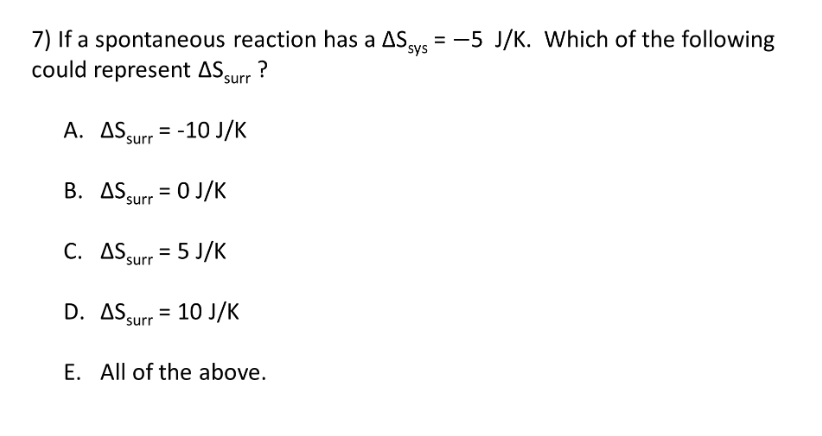
2nd and 3rd Laws of Thermodynamics
E. All of the above

2nd and 3rd Laws of Thermodynamics
B. Δsurr > 0 J/K
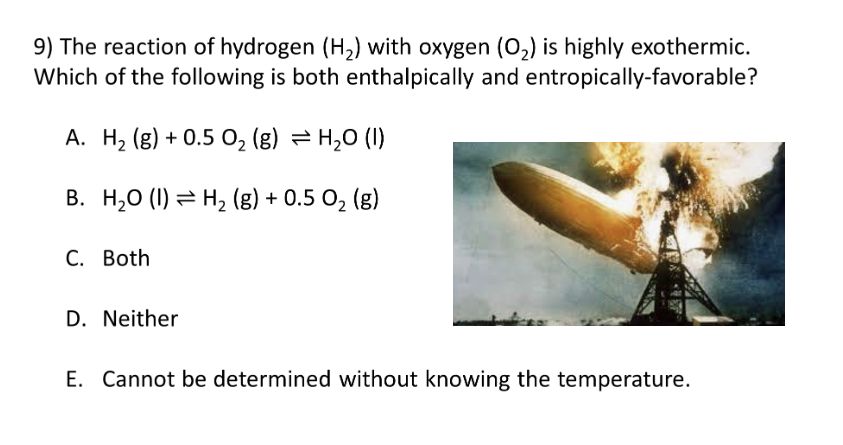
2nd and 3rd Laws of Thermodynamics
D. Neither

2nd and 3rd Laws of Thermodynamics
C. K (s) at 0 K
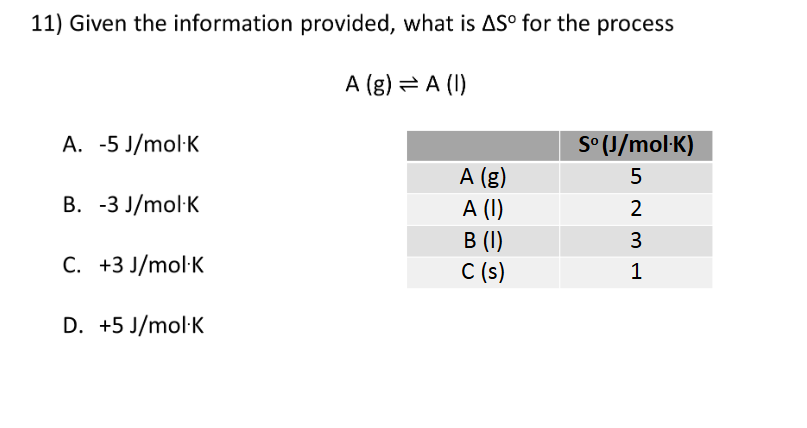
2nd and 3rd Laws of Thermodynamics
B. -3 J/mol*K
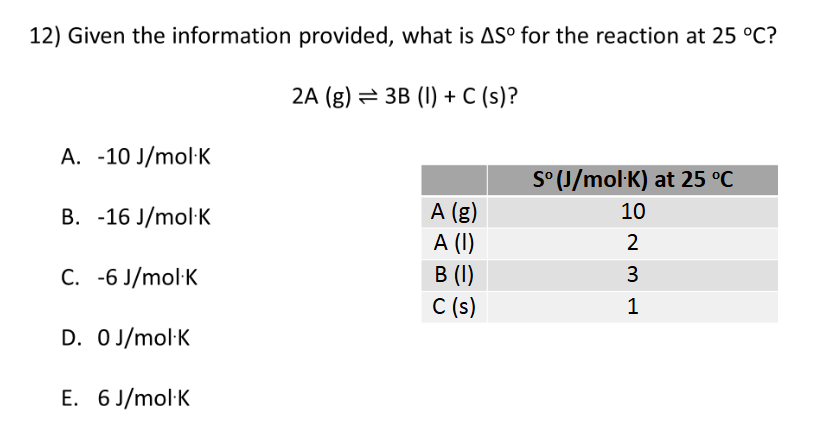
2nd and 3rd Laws of Thermodynamics