Unit A: Mix of Flow and Matter
1.0 fluids are used technological devices and everyday materials
Lab Safety and WHMIS



a yellow upside-down triangle means caution
an orange diamond means a warning
a red octagon means danger
Workplace
Hazardous
Materials
Information
System

⭐WHMIS safety symbols
Safety
Data
Sheet
Recall:
Matter: takes up space, has mass
Fluid: liquid or gas, no fixed shape, can flow
Use of fluids
slurries: a mixture of a liquid and a solid in which the liquid is used to move the solid (ex. hosing doggy doo off the driveway
Fluids become solids
Many solids are prepared as fluids (ex. glass, cement)
Fluids can hold other materials acting as a binder (toothpaste, glitter glue)
Properties of fluids
density
buoyancy
viscosity
compressibility
concentration
2.0 The particle model of matter can explain the properties of mixtures and fluids.
Pure substances and mixtures
Pure substance: elements that consist of only 1 kind of atom (ex. oxygen)
Compounds: consist of two or more elements chemically bonded in specific ratios
Mixtures: consist of two or more pure substances, not chemically bonded, can be separated
Types of mixtures:
Homogenous mixture- 2 or more components appear as one (homo) this mixture is a solution (particles are evenly distributed)
Heterogenous mixture- this mixture is a mechanical mixture (different components are visible) ex. mixed veggies
Other types of mixtures:
suspension- when particles are suspended or hanging in a mixture they will settle out. (ex. muddy water) When the dirt particles were moving and hanging, it was suspended
colloid- a mixture so well mixed, that it would not separate (ex. jello)
Classifying matter
Concentration and solubility
Dissolving- when two or more substances’ particles are intermixed
A solution has two parts:
solute
solvent
Solute- the substance that is dissolved. For example, kool-aid in water. The juice crystal is the solute
Solvent- the substance that does the dissolving. For example, kool-aid in water. Water is the solvent
Soluble- a solute will dissolve in a solvent
Insoluble- a solute will NOT dissolve in a given solvent
Measuring concentration
Concentration- the actual amount of solute in the actual amount of solvent ex. a sugar solution was made with 5g of sugar and 100ml of water
C=M/V Where c is concentration, m is mass, and v is volume. Concentration can also be expressed as a percent or using a weight-volume ratio
Dilute solution- has little solute in large volumes of solvent'
Concentrated solution- a large amount of solute in smaller volumes of solvent
Saturated solution- a solution in which no more solute will dissolve
Unsaturated solution- a solution in which more solute can dissolve
Aqueous solution- a solution in which the solvent is water
The particle model of matter
Everything is made up of particles
The particles are in constant motion
If you add energy, that particle motion will increase, if you remove energy, the particle motion will decrease
there is space between the particles
The PMM explains mixing
Particles are different sizes and when two substances are mixed, the smaller particles fill the spaces between the larger particles. The particle model also states that particles are attracted to each other. However, sometimes particles are more attracted to themselves and they will not mix with another kind of particle
Factors affecting rates of dissolving
The 3 factors that affect the rate of dissolving are:
Temperature. When the temperature is increased, the rate of dissolving increases
Agitation. Agitating, or shaking will increase the rate of dissolving
Surface area. Increasing surface area will increase the rate of dissolving. For example, crushing a compact small tablet into a loose powder will help the particles mix.
Helpful questions
1. Where is WHMIS found?
2. What are fluids?
3. What does matter do?
4. What do the particles of substance do? What is the Particle Model of Matter?
5. What are hazard symbols? Where are they found? What colors are they? What do the shapes mean?
6. Know the WHMIS 2015 symbols. What do they mean?
7. What are the properties of fluids?
8. How are fluids used? Can you give two examples?
9. Know (in general) the Lab Safety Rules Know why they're important.
3.0 The particle model of matter can explain the properties of gases and liquids.
Viscosity- the internal friction of a substance’s particles. The more friction, the more viscous a substance is.
a more viscous fluid flows slowly, and a less viscous fluid flows quickly
Viscosity is affected by temperature. Increased temp= lower viscosity. Decreased temp= higher viscosity
Calculating flow rates
R= D/T Where r is flow rate, d is distance, and t is time
ex. An oil slick flowed 10m downhill in 5 min, What’s the flow rate?
r=d/t
r=10m/5min
r=2m/min
Density of a fluid
Density- the amount of matter in a given volume
Volume- the amount of space an object occupies LxWxH (or area of base x h) meters x meters x meteres= m3 or meters cubed
In liters of cubic units 1m= 1cm3 of water
Floating- when a less dense substance is in a more dense substance, it floats. (ex. oil on top of water)
Sinking- when a more dense substance is in a less dense substance it will sink (ex. a rock in the water)
D=M/V Where d is density, m is mass (g), and v is volume (ml for liquids and cm3 for solids)
Cheaters triangle
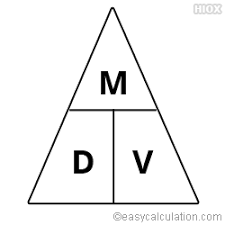 it works like this: cover the variable you are trying to solve for
it works like this: cover the variable you are trying to solve for
D=M/V
V=M/D
M= (D) (V)
Density, temperature, and buoyancy
Density is a constant provided the temperature remains the same
Density and Concentration
The, more concentration there is, the more particles there are, which means it is dense
Buoyancy
Buoyancy- the tendency for an object to float
Buoyant force- the force in fluids that act against gravity
The larger upward buoyant force=smaller downward force of gravity= float
Smaller buoyant force= larger downward force of gravity= sink
N upward buoyant force (neutral) =N downward force of gravity= hover
Application of buoyancy: hot air balloons
A burner heats the air inside, and since hot air rises, the balloon will rise. To lower the hot air balloon, flaps are opened to cool the air inside, and since cool air sinks, the balloon will descend.
Plimsoll line
Plimsoll line- a line on the bow or stern of boats that determines how much cargo can be safely loaded depending on the waters’ temperature and concentration
Compressibility and Pressure
gases are most easily compressed
liquids compress very little
solids hardly, if at all, compress
Pressure- is a measure of force distributed over an area
P=F/A Where f is force (Newtons), A= area (meters)
The unit of pressure is Pascal (Pa)
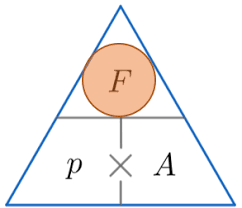
Therefore P=N/M2 =Pa
Pascals law
In an ENCLOSED SYSTEM, pressure is transmitted equally in all directions
Hydraulic- closed fluid is a liquid
Pneumatic-enclosed fluid is a gas
Helpful questions (section 3)
1. What is a measure of a substance's friction between its particles? How does it affect flow?
2. What does viscous mean?
3. How does temperature affect viscosity and density?
4. Why do people in cold climates plug in their cars?
5. Can you calculate density?
6. What is buoyant force? What does it describe?
7. What's the purpose of a Plimsoll line?
8. What is pressure? How is it defined? What is the unit used to measure pressure?
9. What is the difference between hydraulic and pneumatic devices?
10. What are the advantages of a pneumatic drill?
11. What is Pascal's Law? When does it not hold true?
12. How does a hot air balloon work?
Answers
Viscosity. The more viscosity the more friction and vice versa
The internal friction of a substance's particles
higher temperature= lower viscosity. lower teperature= higher viscosityhigher temp= lower density. Lower temp=higher density
Because the oil will freeze
D=M/V
the force in fluids that acts against gravity describes the force of pushing up
the line on a ship that determines how much cargo can safely be loaded depending on the water temperature and concentration
is a measure of force distributed over an area. Force divided by area. Pascal’s
hydraulic uses liquid as the enclosed fluid. pneumatic uses air
at reasonable cost and safety. Compressed air is cheap and safe because the air inside the system will not create sparks
in an enclosed system, pressure is transmitted in all directions. when the system is not enclosed
A burner heats the air inside, and since hot air rises, the balloon will rise. To lower the hot air balloon flaps are opened to cool the air inside, and since cold air sinks, the hot balloon will descend
4.0 – Many technologies are based on the properties of fluids.
Technologies Based on Solubility
Hydrophobic- water-hating
Hydrophilic- water-loving
Most detergents are liquids or powders. They are water-soluble.
Typical components of detergent
surfactant- cleans fabric, soap
Prevents clumping
Suspension agent- prevents re-attaching
How detergent works
#1 There is dirt and grease on the fabric
#2 The mixture of water, detergent, and clothes is agitated. Dirt breaks off the clothes
#3 Surfactants surround the dirt so they don’t re-attach. It makes an emulsion that can be drained
4.0 – Many technologies are based on the properties of fluids.
Submarines
use a ballast tank to change the density of the submarine so that it can dive, surface, and float
Self
Contained
Underwater
Breathing
Apparatus
Pump- forces a liquid to go against the force of gravity
Valves- control the rate of flow, pressure, and volume
Pipeline Pig-
looks like a bullet
has ridges
traveling through pipelines keeping them clear
Unit A Questions
1. What does WHMIS stand for?
2. Where and why is WHMIS used?
3. What are the characteristics of a fluid?
4. Can you give an example of a slurry?
5. Into what two groups can all matter be divided?
6. What is a suspension?
7. What is another name for a solution?
8. What is a solvent? What is a solute?
9. How does temperature affect solubility?
10. How is concentration calculated?
11. What are the four tenets of the Particle Model of Matter?
12. What is viscosity?
13. What effect does temperature have on viscosity?
14. What is density?
15. What is Pascal's Law? How does it work?
16. What can control the rate of flow?
17. What is the difference between a hydraulic and a pneumatic system?
18. What is a pig?
19. What is called when nitrogen gas is trapped in a diver's blood?
20. What are the three main activities of a submarine?
Answers
Workplace hazardous materials information system
workplaces, they are there to help protect people who use harmful materials at work density,
buoyancy, viscosity, compressibility, and concentration
hosing doggy doo doo off the driveway
pure substances and mixtures
when particles are suspended or hanging in a mixture
homogeneous mixture
the part that dissolves the part that does the dissolving
a higher temperature = a higher rate of solubility. lower temperature = a lower rate of solubility
c= m/v
Everything has matter. Mater is always moving. An increase in energy = the increase of a particle's motion. decrease in energy = a decrease in the particle's motion. particles have space between them
the higher temperature = lower viscosity, the lower temperature = higher viscosity
the amount of matter in a given volume
Pascal's law states that in an enclosed system, pressure is equally transmitted in all directions
Valves
In a hydraulic system, the enclosed system fluid is compressed liquid, and in a pneumatic the enclosed fluid system is compressed air
looks like a bullet, has, ridges, and moves through pipes to keep them clear
decompression sickness-” the bends”
floating, diving, and resurfacing