Microbial Biotechnology Final
1/422
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
423 Terms
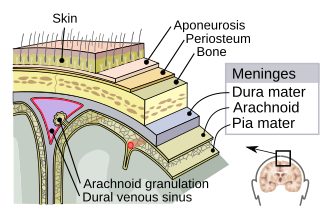
Meninges
The meninges are the three protective membranes that surround the brain and spinal cord. Their main functions are to:
Protect the central nervous system (CNS)
Provide a supportive framework for blood vessels
Contain the cerebrospinal fluid (CSF), which cushions the brain and spinal cord
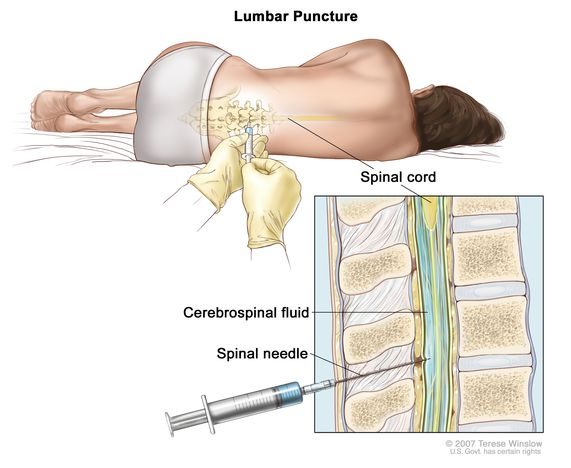
How is cerebrospinal fluid (CSF) collected, and why is it collected?
A spinal tap can be used to extract CSF by inserting a needle below the the spinal cord termination
The CSF can be used to help diagnose serious infections, such as meningitis.
What are the components of the meninges and what are their functions?
Dura mater: The outermost and thickest layer, directly beneath which protects the central nervous system
Arachnoid mater: A spiderweb-like middle layer that has connective tissue projections that attach to your pia mater
Pia mater: The innermost layer (similar to shrinkwrap) that contain cerebrospinal fluid
Cerebrospinal fluid (CSF)
A clear bodily fluid that occupies the subarchanoid space and the ventricular system around and inside the brain. The brain essentially “floats” acting as a “cushion” or buffer for the cortex, providing a basic mechanical and immunological protection to the brain inside the skull
How does the cerebrospinal fluid (CSF) provide immunological protection?
CSF is continuously produced and circulates through the ventricles, central canal, and subarachnoid space.
This flow helps flush out waste, toxins, and potential pathogens, preventing accumulation near brain tissues.
The glymphatic system (a waste-clearing pathway using glial cells) works with CSF to remove metabolic waste and microbes.
What are the structures of the central nervous system?
Brain
Cerebrum controls voluntary muscles, perceptions, and “thinking”
Cerebellum controls many involuntary body movements (i.e. arm swinging when walking)
Brain stem controls breathing, heart rate, blood pressure
Spinal Cord
Extend from the brain stem to the lumbar region
Send motor commands from the brain to the body, send sensory information from the body to the brain, and coordinate reflexes
What are the structures of the peripheral nervous system?
Cranial nerves extend from the brain through holes in the cranial bones
Spinal nerves extend from the spinal cord through gaps between the vertebrae
Three types of nerves:
Sensory nerves - carry signals toward the CNS
Motor nerves - carry signals away from CNS
Mixed nerves - mixed of both carry and toward CNS
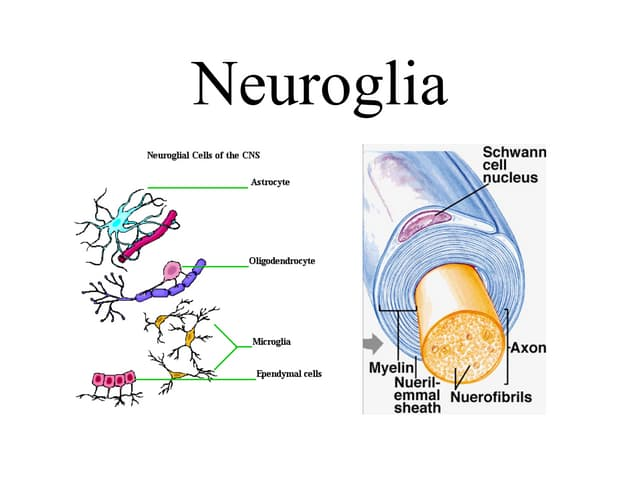
What are the two basic types of nervous system cells?
Neuroglia
Neurons
Neuroglia
Provide support, insulation, nutrients, phagocytize microbes
Includes astrocytes, microglia, oligodendrocytes (CNS) and Schwann cells (PNS)
Neurons
Carry nerve impulses
Nucleus lies in a region called the cell body
A collection of many neurons’ cell bodies is called a ganglion (located outside of CNS)
Dendrites and axons extend from the cell body
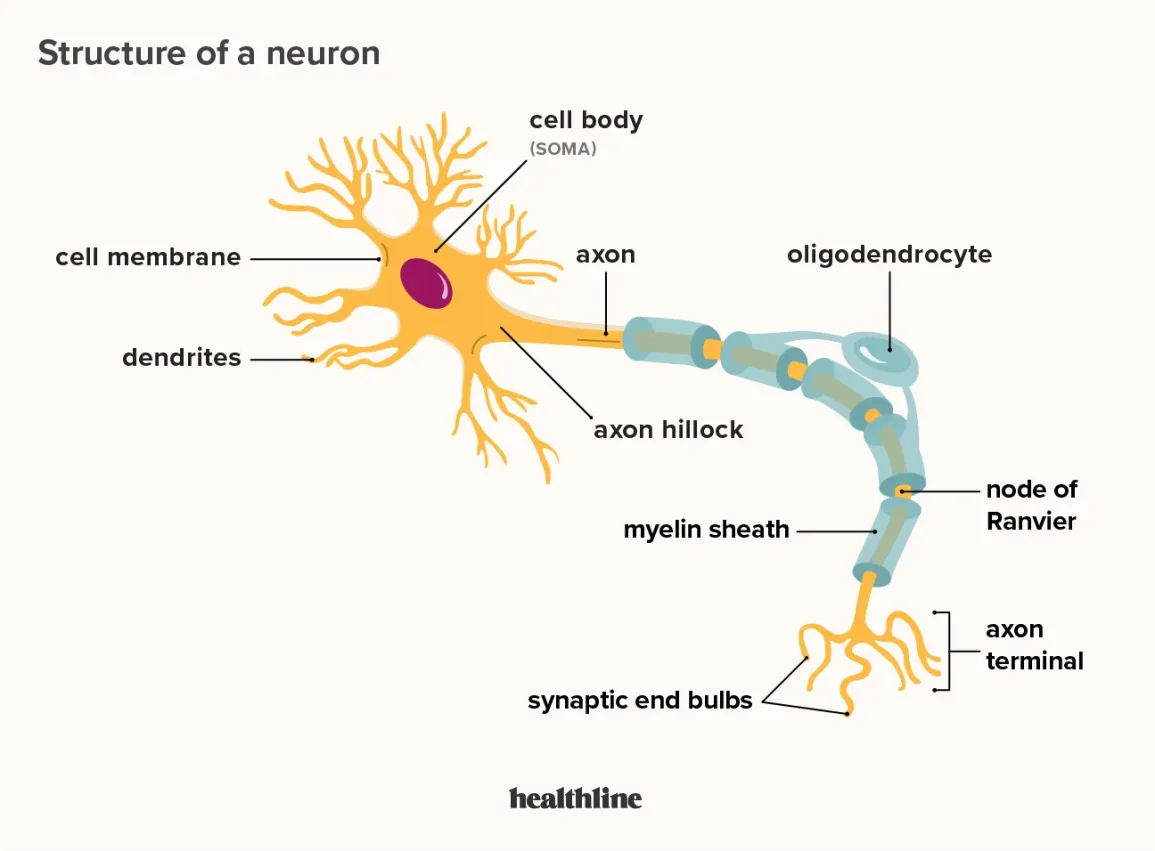
_____ receives signals and ____ transmits signals away from the cell
__Dendrites__ receives signals and __axons__ transmits signals away from the cell
Astrocytes
Maintain blood-brain barrier (protects brain from harmful substances in blood)
Regulate ion and neurotransmitter levels in the extracellular space
Provide nutritional and structural support to neurons
Help in repairing injuries (form glial scars)
Connect neurons to blood supply via their end-feet
Mircoglia
Act as the immune cells of the CNS (like macrophages)
Perform phagocytosis: remove debris, damaged neurons, and pathogens
Play roles in inflammation and brain injury response
Oligodendrocytes
Produce myelin in the CNS (wrap around axons to form myelin sheath)
Each oligodendrocyte can myelinate multiple axons at once (like an octopus)
Schwann Cells
Produce myelin in the PNS
Each Schwann cell myelinates only one axon segment
Also assist in axon regeneration after injury (unique to PNS)
What are the structures of the neuron?
Soma: The control center of the neuron. It contains the nucleus and organelles, integrates incoming signals, and produces neurotransmitters.
Dendrites: Receive chemical signals from other neurons and convert them into electrical signals (graded potentials).
Axon: Long projection that conducts electrical impulses (action potentials) away from the soma to the axon terminals.
Myelin Sheath: Fatty layers that wrap around the axon, insulating it, and increasing the speed and efficiency of action potential transmission along the axon.
Terminal buttons: When an action potential arrives, vesicles (fill with neurotransmitters) are released into the synapse to communicate with the target cell.
Schwann cell: A type of glial cell in the Peripheral Nervous System (PNS) that produces the myelin sheath.
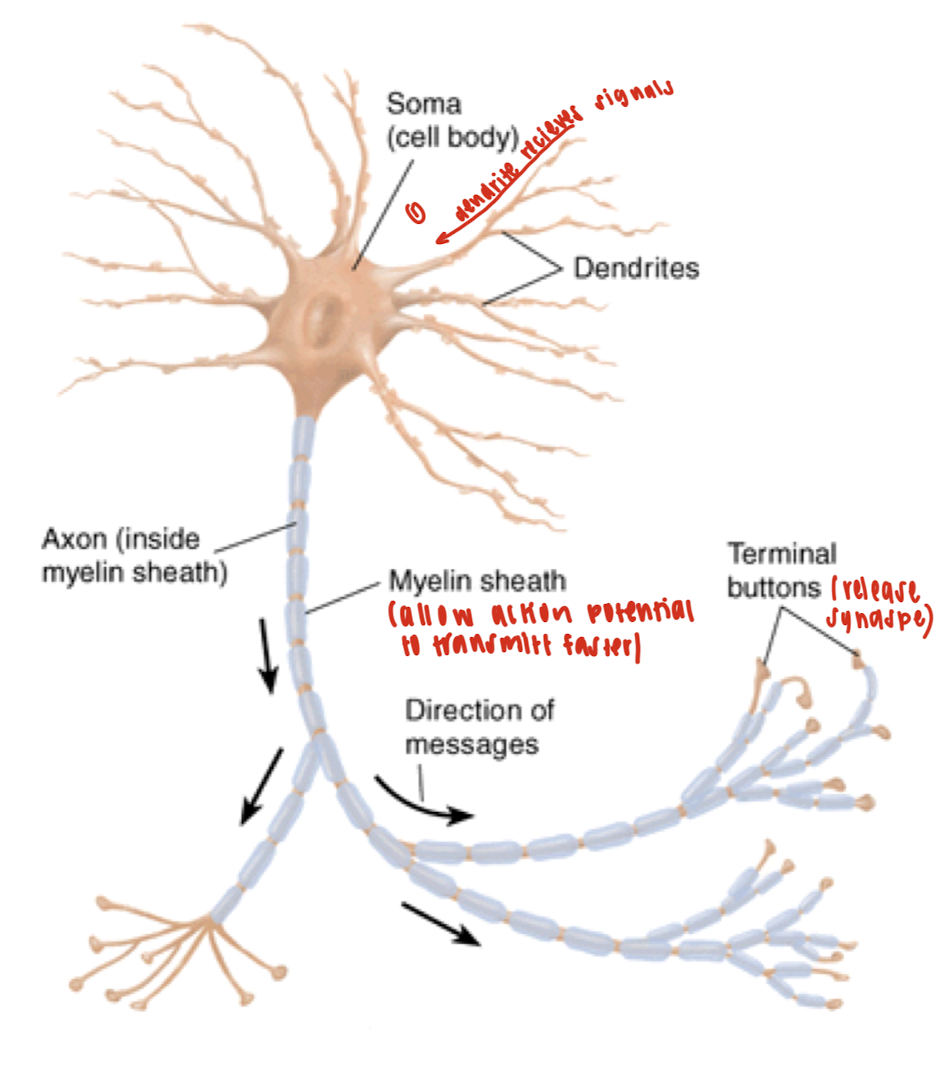
What is the flow of electricity in a neuron?
Dendrites receive a signal.
The signal is integrated in the soma.
If threshold is reached, an action potential fires down the axon.
The signal reaches terminal buttons, which release neurotransmitters.
Neurotransmitters cross the synapse.
They bind to receptors on the postsynaptic cell (target cell).
The target cell responds by firing its own signal or carrying out a function.
The central nervous system is an ____ environment
Axenic
No normal microbiota
How do pathogens access the central nervous system?
Breaks in the bones and meninges
Medical procedures
Traveling in peripheral neurons to the CNS
Compromised blood-brain barrier (BBB)
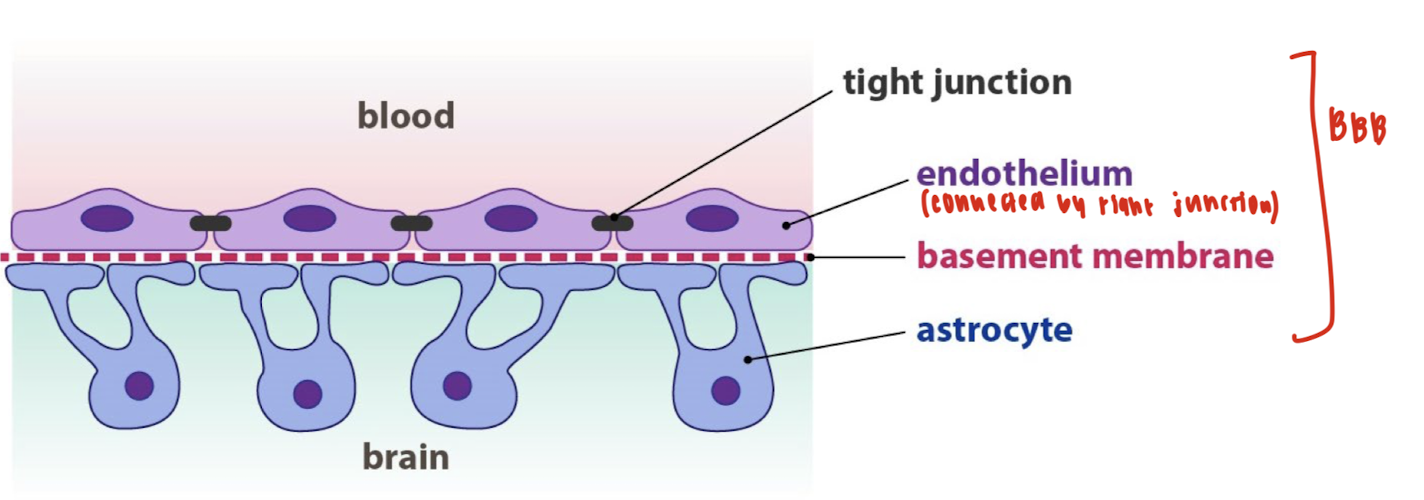
What is the blood-brain barrier (BBB)?
A filter mechanism that allows only selected substances into the brain.
Advantages: block entering of harmful bacteria.
Disadvantages: block entering of antibiotics
What is the layer of the blood-brain barrier (BBB)?
A highly specialized endothelial cells (EC) layer comprising the blood-brain barrier (BBB) and partitioning the blood and brain interstitial fluid
The blood-CSF barrier (BCSFB) with the choroid plexus epithelium which secretes the specialized cerebral spinal fluid (CSF) into the cerebral ventricles
The arachnoid epithelium separating the blood from the subarachnoid CSF
What are the signs and symptoms of bacterial meningitis?
Increased number of white blood cells (WBC) in the cerebrospinal fluid (CSF)
Sudden high fever and severe meningeal inflammation
Inflamed cranial meninges – severe headache, vomiting, pain
Inflamed spinal meninges – stiff neck, altered muscle control
Infection of the brain, or meningoencephalitis, can result in behavioral changes, coma, and death
All may develop rapidly
What does a high MAP score mean?
A MAP score of more than 130 mmHg means that more blood flow results in pressure build-up, leading to headache and pain sensitivity.
What does a low CPP score mean?
An increase in ICP results in a decrease in CPP, causing blood flow to decrease and resulting in ischemia and failing
What are the main 5 species that causes 90% of the bacterial meningitis cases?
Streptococcus pneumoniae
Neisseria meningitidis
Haemophilus influenzae
Listeria monocytogenes
Streptococcus agalactiae
What pathogen is the leading cause of adult bacterial meningitis?
Streptococcus pneumoniae
What pathogen is the leading cause of bacterial meningitis prior to vaccination?
Haemophilus influenzae
How does Neisseria meningitidis cause bacterial meningitis?
N. meningitidis due to certain virulence factors such as fimbria, capsule, and lipooligosaccharide
What pathogen is the leading cause of bacterial meningitis in fetuses, pregnant women, and immunocompromised individuals?
Listeria monocytogenes
What pathogen is the leading cause of bacterial meningitis in newborns?
Streptococcus agalactiae can be acquired during birth
What is the transmission mode of each of the five species that causes bacterial meningitis?
Bacterial Species | Transmission Mode | Common Setting/Population |
|---|---|---|
Neisseria meningitidis | Respiratory droplets | Teens, young adults in close quarters |
Streptococcus pneumoniae | Respiratory droplets | Infants, elderly |
Haemophilus influenzae (Hib) | Respiratory droplets | Unvaccinated children |
Listeria monocytogenes | Contaminated food; vertical | Pregnant women, immunocompromised |
Group B Streptococcus | Birth canal (vertical) | Newborns |
True/False: S. pneumoniae is present in the throat of 75% of humans without causing harm
True
What is the only form of meningitis that can become an epidemic?
Meningococcal meningitis
Which strain(s) of meningitis are vaccineable?
Streptococcus pneumoniae
Neisseria meningitidis
Haemophilus influenzae type B
They are vaccineable because they have a polysaccharide capsule — a major virulence factor that can be targeted by the immune system.
What food(s) should be avoided by high-risk individuals against listeriosis?
Milk
Cheese
Undercooked meat
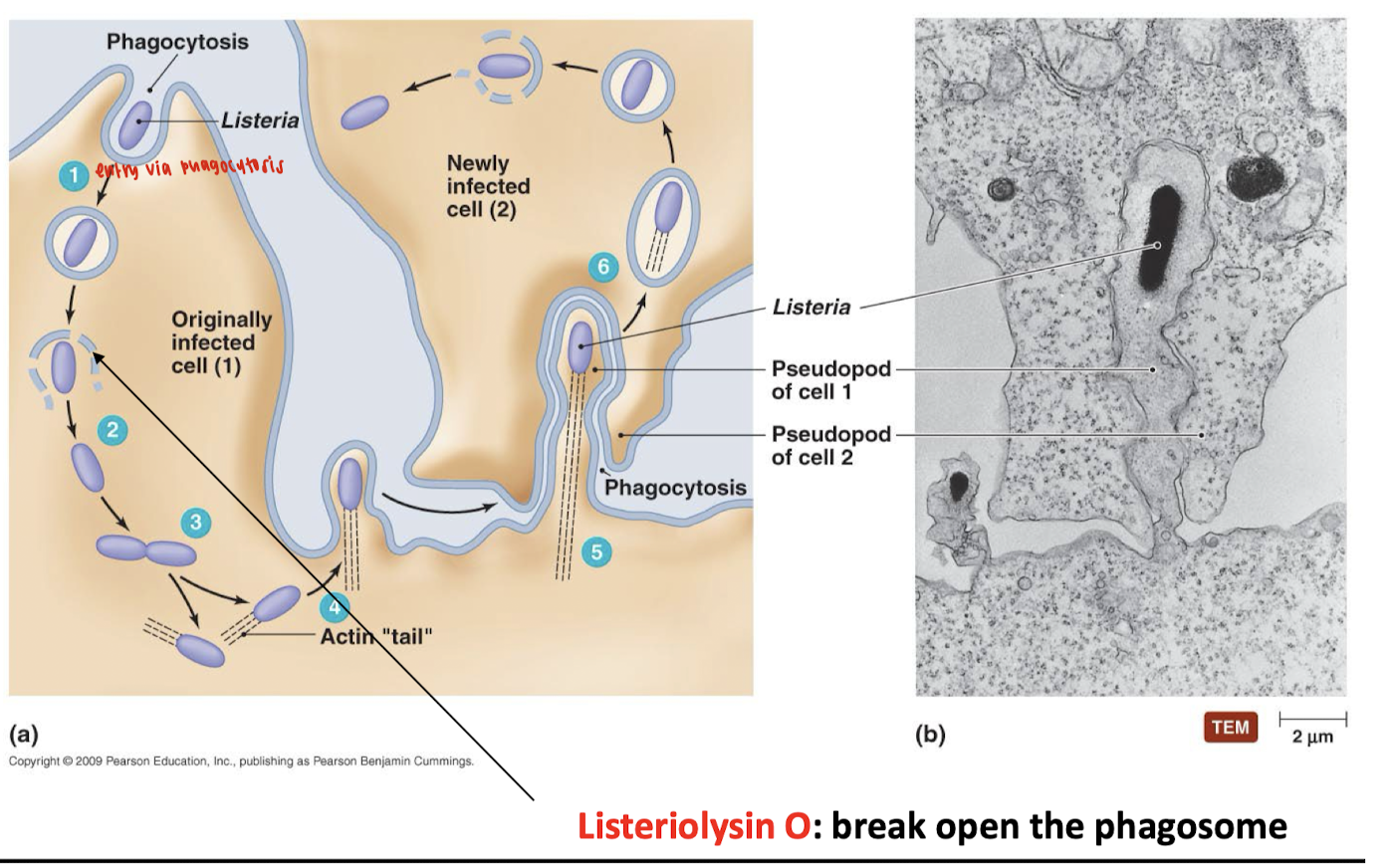
What is the life cycle of Listeria monocytogenes?
1. Entry via phagocytosis
Listeria enters the host cell through phagocytosis, typically by macrophages or epithelial cells.
The bacterium is enclosed in a phagosome.
2. Escape into the cytoplasm
Listeria produces Listeriolysin O, a pore-forming toxin.
This toxin breaks open the phagosome, releasing Listeria into the host cell's cytoplasm.
3. Intracellular replication
Once in the cytoplasm, Listeria replicates safely, avoiding immune detection.
4. Actin-based motility
Listeria hijacks the host's actin cytoskeleton to form an actin "tail".
This tail propels the bacterium through the cytoplasm toward the cell membrane.
This movement pushes the bacterium into the membrane, causing Cell 1 to form a protrusion, or “pseudopod,” that extends into a neighboring cell (Cell 2).
5. Cell-to-cell spread
Listeria pushes against the host membrane to form membrane protrusions.
The protrusion formed by Cell 1 invaginates into Cell 2, which engulfs the tip of the protrusion via phagocytosis by the next cell.
6. Repeat of infection cycle
Once inside the new host cell, Listeria escapes the phagosome using Listeriolysin O and repeats the cycle.
What is the function of listeriolysin O?
Mediate escape from phagosomes by opening up the phagosome, allowing the bacterium to replicate within the host cell cytosol
What are the basic features of Listeria monocytogenes?
Gram (+)
Found in soil, water, mammals, birds, and insects
Enters via contaminated foods and drinks
Causes meningitis in pregnant women, fetuses, newborns, elderly, and immunocompromised patients
Rarely pathogenic in healthy adults
Causes listeriosis (a foodborne illness)
What are the characteristics of the group D Streptococci: Streptococcus pneumoniae?
A.K.A. “Captain of the men of death”
Discovered by Louis Pasteur
5th leading cause of death in USA
Follows influenza being the only infectious disease among the top 10
150,000 ~ 270,000 cases of pneumococcal pneumonia and 2600 ~ 6200 cases of pneumococcal meningitis occur each year in the USA
Part of lungs, sinuses, and middle ear microbiota
Endogenous species
Carries asymptomatically in the upper respiratory tract by 5-70% of the general population
Lowest carriage rates among adults living with NO children and highest among the population with adults and children in close proximity when the weather is cold
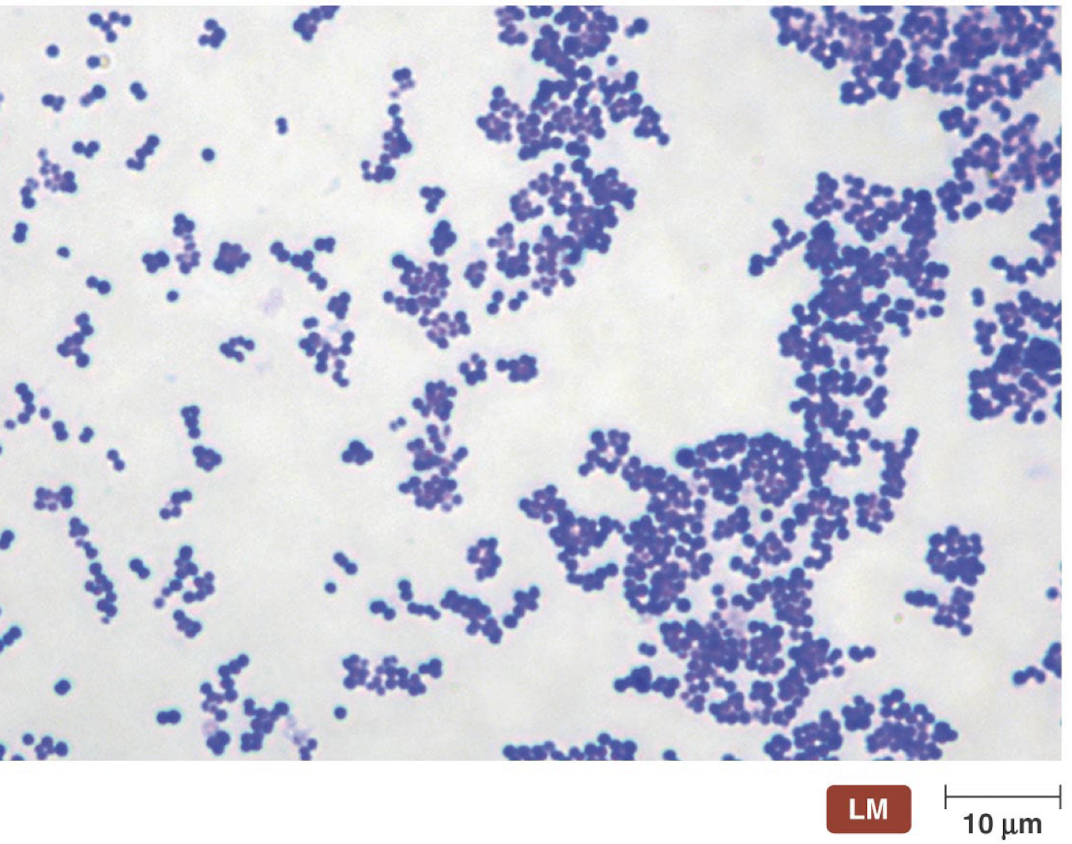
Endogenous disease
A disease caused by an infectious agent that is already present within the body before the onset of symptoms and not acquired de novo from other atients with an active disease
What are the virulence factors of Streptococcus pneumoniae?
Virulence Factor | Function | Role in Disease |
|---|---|---|
Neuraminidase | Thins mucous secretions | Enhances bacterial access to lung tissue |
IgA Protease | Cleaves secretory IgA, IgA, and IgM | Prevents immune-mediated clearance at mucosal surfaces |
Extracellular Cysteine Protease | Converts kininogens to kinins | Causes hypotension, fever, pain, and increased vascular permeability (inflammatory mediator) |
C Substance | Teichoic acid component | Combines with beta-globulin, may trigger immune responses |
F Substance | Cross-reacts with host cell antigens | Potential molecular mimicry, aiding immune evasion |
Pneumolysin | Toxin that interferes with lysosomal digestion | Prevents destruction inside phagocytes; damages host cells |
Phosphorylcholine | Anchored in bacterial cell wall | Binds receptors on lung, meningeal, and blood vessel cells to trigger endocytosis and entry into host cells |
How many capsule serotypes of Streptococcus pneumoniae are pathogenic?
Type 1 to 9 (~10%)
Which type of pneumococci are the most dangerous?
Type 3 pneumococci are the most dangerous because it has the largest capsule, leading to pneumococcal pulmonary abscesses
Streptococcus pneumoniae vaccine is a ____ vaccine.
multivalent
A multivalent or polyvalent vaccine is designed to immunize against two or more strains of the same microorganism, or against two or more microorganisms
Which structure protects Streptococcus pneumoniae from being phagocytosed by polymorphonuclear leukocytes (PMNs)?
Capsule
T/F: Pneumococci are protected from phagocytosis by PMNs due to their capsule.
True
Why is pneumococci the most common community-acquired pneumonia, despite it not being an epidemic disease
Pneumococcal pneumonia is not typically spread in outbreaks; instead, it often develops when pneumococci already present in a person's upper respiratory tract migrate into the lungs and replicate in the alveoli, especially when immune defenses are weakened. Since the immune system is impaired, it diminishes the antibody responses or the polymorphonuclear leukocytes’ function, and increases the aspiration of respiratory secretions will increase the incidence and severity of pneumococcal pneumonia
Which species of Neisseria are pathogenic?
N. gonorrhoeae and N. meningitidis
What are the characteristics of Neisseria?
Gram (-)
Aerobic
Diplococci (kidney bean)
Non-motile
Non-endospore forming
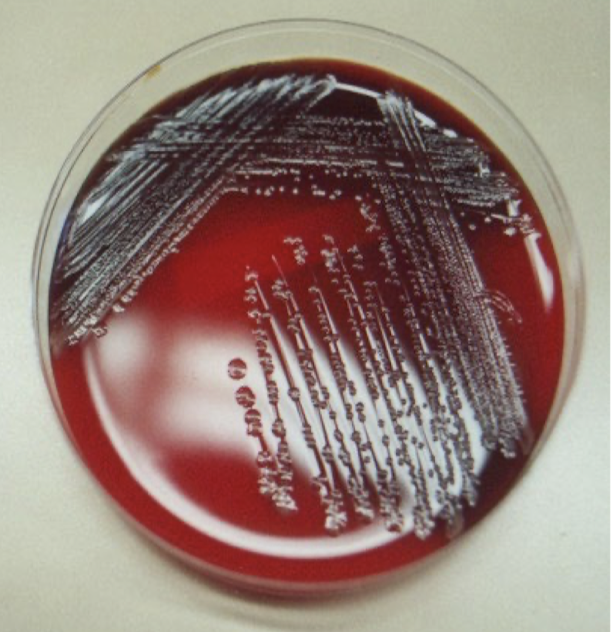
How is Neisseria menigtidis cultured for testing?
Cerebrospinal fluid (CSF) samples are extracted and plated on blood/chocolate agar
CSF should not be refrigerated
Cultured sample at 35C
Meningococci usually form gray or yellowish colonies
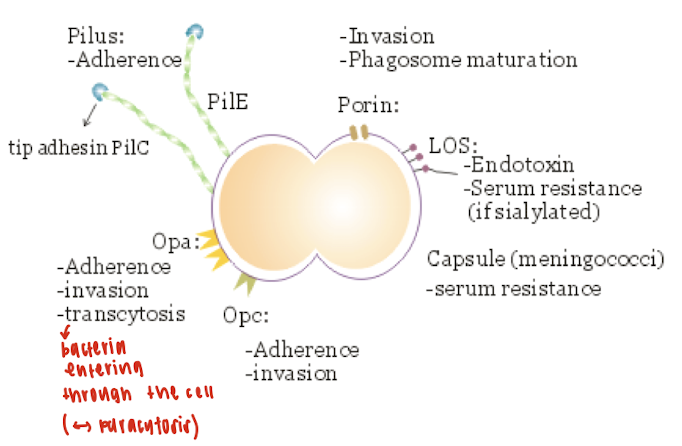
What are the virulence factors of Neisseria menigtidis?
Capsule
Pili
Lipooligosaccharide
Outer membrane proteins
Which Neisseria meningitidis serogroups are infectious/pathogenic? And where are they prevalent?
Type A:
Highly prevalent in sub-Saharan Africa, particularly in the "meningitis belt" (Senegal to Ethiopia)
Also seen worldwide during epidemics
Most associated with large outbreaks and epidemics
Type B:
Common in Europe, North America, and Oceania
Often causes sporadic cases, not large outbreaks
Difficult to develop vaccines against due to poor immunogenicity of its capsule
Type C:
Found in North America, Europe, and parts of Asia
Often associated with localized outbreaks, especially in schools or military camps
Vaccines available and effective
Type W135:
Emerged in Africa, the Middle East, and parts of South America
Known for causing severe disease and increased mortality rates
Spread notably during Hajj pilgrimages
Type Y:
Increasing in North America and Europe
Tends to cause respiratory and bloodstream infections rather than meningitis alone
What are the three vaccines that are licensed against Neisseria meningitidis?
Univalent vaccine directed against type A
Univalent vaccine directed against type C
Polyvalent vaccine directed against types A, C, W-135, and Y
Note: There are no univalent vaccines for type B because it susceptibility in gene shifts
What is the function of pili in Neisseria meningitidis?
Pili mediate adherence to host cells and may play a role in initial colonization.
Why are pili considered important in the infection process?
Because they are an important "initial sneaking force" that help bacteria spread and adhere.
How does the role of N. meningitidis pili in pathogenesis compare to that of N. gonorrhoeae?
The role is considered less important than the pili of N. gonorrhoeae in disease.
What is lipooligosaccharide and why is it significant in Neisseria meningitidis infections?
LOS is highly endotoxic and contributes to the severity of meningococcal septicemia.
What mechanism allows lipooligosaccharide to exert toxic effects?
The blebbing mechanism—membrane vesicles (blebs) containing LOS are released from the bacterial cell and cause toxic effects in the host.
______are the only natural carriers for N. meningitidis and the highest rate oral and nasopharyngeal carriage rates are highest for ______.
__Humans__are the only natural carriers for N. meningitidis and the highest rate oral and nasopharyngeal carriage rates are highest for ___school age children and young adults___.
What is the initial site of infection for N. meningitidis and how does it colonize the site?
The initial site of infection appears to be the nasopharynx, where the meningococci adhere via pili to squamous epithelial cells and then enter the blood.
What are the characteristics of Haemophilus influenzae?
Small rod
Gram (-)
Most commonly associated with pediatric patients before the introduction of H. influenzae type B (HIB) vaccine
_______ is well recognized as the etiologic agent of the sexually transmitted disease, called chancroid
Haemophilus ducreyi
Which Haemophilus species commonly colonize the upper respiratory tract early in life?
Haemophilus parainfluenzae and nonencapsulated H. influenzae.
What types of infections are typically caused by nonencapsulated H. influenzae?
Local infections such as otitis media, sinusitis, and bronchitis.
How common is disseminated disease caused by nonencapsulated H. influenzae?
Relatively uncommon
Where is encapsulated H. influenzae typically found in the body?
It is uncommon in the upper respiratory tract or found only in very small numbers but common in unvaccinated children.
In which population is encapsulated H. influenzae a common cause of disease?
In unvaccinated children.
Haemophilus species
“Blood-loving”
Require one or two growth factors present in blood
X factor (Hemin, hematin, or other tetrapyrroles)
V factor ( NAD, NADP)
10/15 pathogenic species
Major pathogen best known for its ability to cause life-threatening diseases in young children
Meningitis and epiglottitis
What are the key virulence factor of Haemophilus influenzae?
The antiphagocytic capsule
LOS (lipooligosaccharide)
Pili
IgA protease
What are the characteristics of Haemophilus influenzae capsule?
6 capsule types (a-f)
>95% of the disease was caused by type B before the introduction of HIB vaccine
Currently serotypes C & F and noncapsulated H. influenzae are responsible for most H. influenzae disease
Non-typeable H. influenzae strains
H. influenzae strains associated with localized infections that do not produce a polysaccharide capsule
What are the characteristics of Haemophilus influenzae lipooligosaccharide?
Naturally rough LPS (immunogenic antigen) (it doesn’t have a smooth sugar chain on it)
Makes it easier for the immune system to detect, but also more dangerous because it elicits of strong immune system response
Core polysaccharides are highly variable and subject to phase variation
turn parts of its LOS on or off to hide or adapt
LOS increases the permeability of the blood-brain barrier because of the high percentage of lipid A (toxic part)
If the bacteria get into your blood (bacteremia), Lipid A can cause sepsis – a dangerous body-wide reaction.
What are the characteristics of Haemophilus influenzae pili?
Express several types of pili that mediate adherence to epithelial cells and erythrocytes
Subject to phase variation
Loss of pili promotes invasion of the epithelium
H. influenzae organisms appear to be piliated when in the nasopharynx, but when they reach the blood and begin to multiply rapidly, strains that cause systemic disease stop expressing pili on their surface
What are the characteristics of Haemophilus influenzae IgA protease?
The protease cleaves human IgA, allowing H. influenzae can stay on mucosal surfaces longer without being cleared by the immune system.
What is the pathogenic process of Haemophilus influenzae?
Adherence:
H. influenzae adheres to the nasal mucosa using:Pili
Possibly other outer membrane proteins
Invasion Route:
Unlike meningococci, which are phagocytosed and transported across epithelial cells,
H. influenzae adheres tightly to damaged mucosa.
It passes through the subendothelium between epithelial cells (not through them), especially in areas of mucosal damage.
Evasion of Host Immune System:
Capsule: The Hib strain has an antiphagocytic capsule that protects it from immune attack.
Capsular release: It releases ~50% of its capsule into the environment.
This acts as a decoy, binding anti-PRP (polyribosylribitol phosphate) antibodies.
This inhibits opsonization and complement activation, helping Hib evade immune clearance.
Haemophilus influenzae is disseminated via ____
respiratory secretions
What are the symptoms Hib?
High fever
Stiff neck
Vomiting
Twitching
Seizures
Other abnormal neurological signs
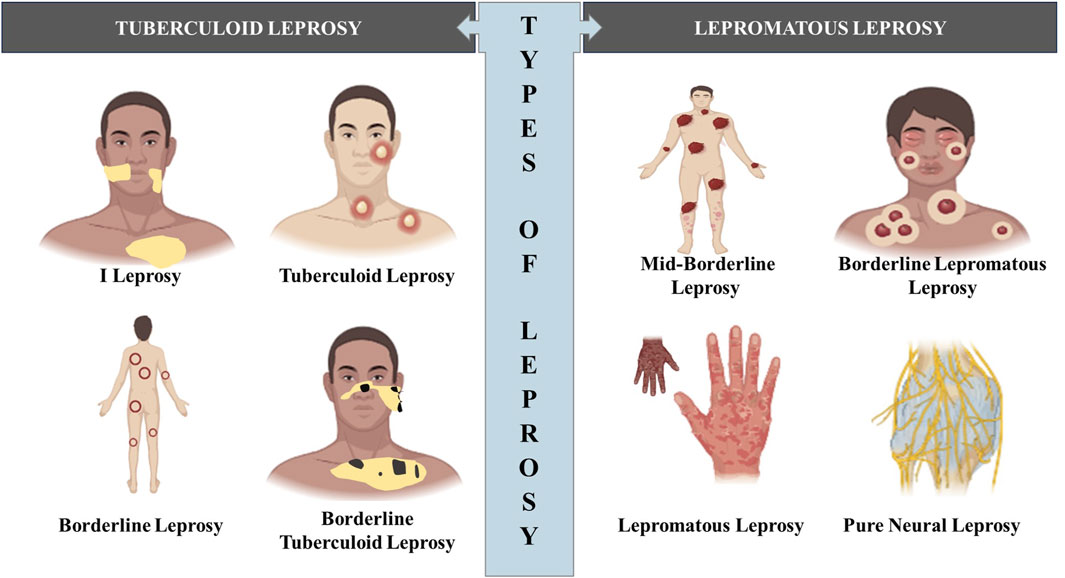
Hansen’s Disease (Leprosy)
Tuberculoid leprosy (minor)
Patients with strong cell-mediated immune responses
Non-progressive form of the disease
Skin that have loss sensation as a result of nerve damage
Lepromatous leprosy (major)
Patients with weak cell-mediated immune responses
Progressive form of disease
Bacterium multiplies in the skin, mucous membranes, and nerve cells, gradually destroying tissues and leading to the progressive loss of facial features, digits (fingers and toes), and other body structures
Mycobacterium leprae
High G+C content
Non-endospore forming
Gram (+) with mycolic acid cell wall
Has NEVER been grown in cell-free lab culture
Need animal model
Armadillo ( 30°C)
Footpads of immunocompromised mice
Grow best at 30°C, showing a preference for cooler regions of the body
How does the mycolic cell wall affect Mycobacterium leprae?
Slow growth rate
Generation time: hours to several days
Protection from lysis once it is phagocytized
Growth within phagocytes
Resistance to gram stain, antimicrobial drugs, and dessications
NEED Acid-fast staining
Where, in the human body, do Mycobacterium leprae target?
Affect peripheral nerves
Reproducing particularly in neuroglia of peripheral nerve endings, cells of the mucous membrane of the nose, and skin cells in the fingers, toes, lips, and earlobes.
Remain alive asymptomatically inside infected cells for 10 - 30 years.
Human leprosy is transmitted via ____
Human leprosy is transmitted only via ___person-to-person contact__
Nasal secretions of patients are loaded with mycobacteria
Infection may occur via inhalation of respiratory droplets → becoming rare
True/False: Industrialized nations quarantine patients with leprosy because the disease is so rarely transmitted and is fully treatable.
False
How is leprosy diagnosed?
Tubercoloid leprosy: a loss of sensation in skin legions
Lepromatous leprosy: disfigurement
How can Mycobacterium leprae be treated?
M. leprae quickly develops resistance to single antimicrobial agents → use of multiple drugs
Treatment takes at least two years and can be lifelong
How can Mycobacterium leprae be prevented?
Partial protection from the tuberculosis vaccine (BCG)
Prevention is primarily achieved by limiting exposure to the pathogen
Prophylactic use of antimicrobial agents when exposure is expected to occur.
What are the signs of botulism?
Food-borne botulism – progressive paralysis of all voluntary muscles
Infant botulism– bacteria grow in the intestines, producing non-specific symptoms
Wound botulism– symptoms like those of food-borne botulism
What pathogen causes botulism?
Clostridium botulinum
Clostridium botulinum
One of seven causative agents
Anaerobic
Gram (+)
Spore-forming bacillus
What is the mechanism of botulism infection?
Source of Toxin:
Botulism is primarily caused by ingesting preformed botulinum toxin.
Less commonly, it can result from:
Wound infection
Germination of ingested spores (especially in infants)
Toxin Production:
Spores of Clostridium botulinum germinate in food (especially in anaerobic, low-oxygen environments like canned food).
The bacteria then produce the neurotoxin.
Risk Factors:
Improper home-canning may fail to sterilize the food or remove oxygen.
Surviving spores germinate and release toxin in the anaerobic canned environment.
Toxin Stability:
The toxin is heat-sensitive, so proper cooking can inactivate it.
However, in improperly cooked food, the toxin remains active.
Absorption and Effect:
Once ingested, the toxin is absorbed from the intestine.
It travels through the bloodstream and affects the nervous system, leading to paralysis.
How does botulism affect the neurons?
The botulinum toxin blocks the release of acetylcholine from the neuron.
Without acetylcholine in the synaptic cleft:
The muscle does not receive the signal to contract.
This leads to flaccid paralysis (muscles become weak and limp).
TLDR botulinum toxin prevents nerve signal transmission to muscles.
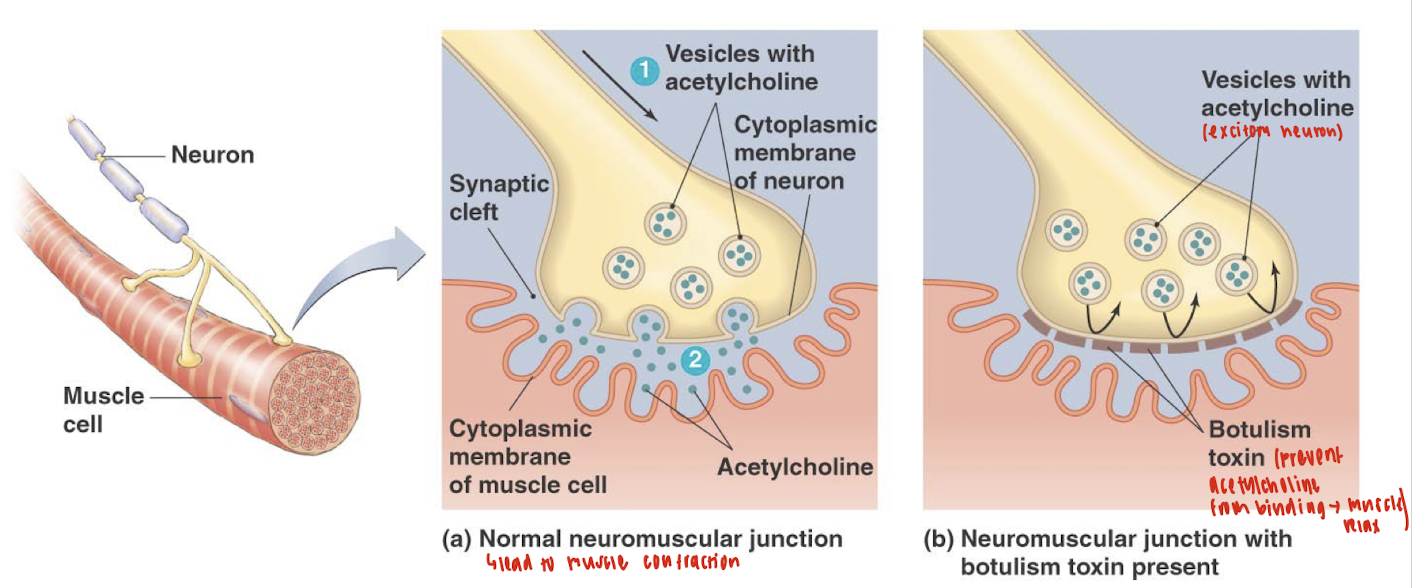
Which form of botulism is the most common in the U.S.?
Infant botulism is the most common form of botulism in the U.S. (~1% death rate)
What are the treatment and prevention methods for botulism?
Treatment:
Washing of the intestinal tract to remove Clostridium
Administration of botulism immunoglobulin
Treatment with antimicrobial drugs
Prevention involves destroying endospores in contaminated food through proper canning techniques
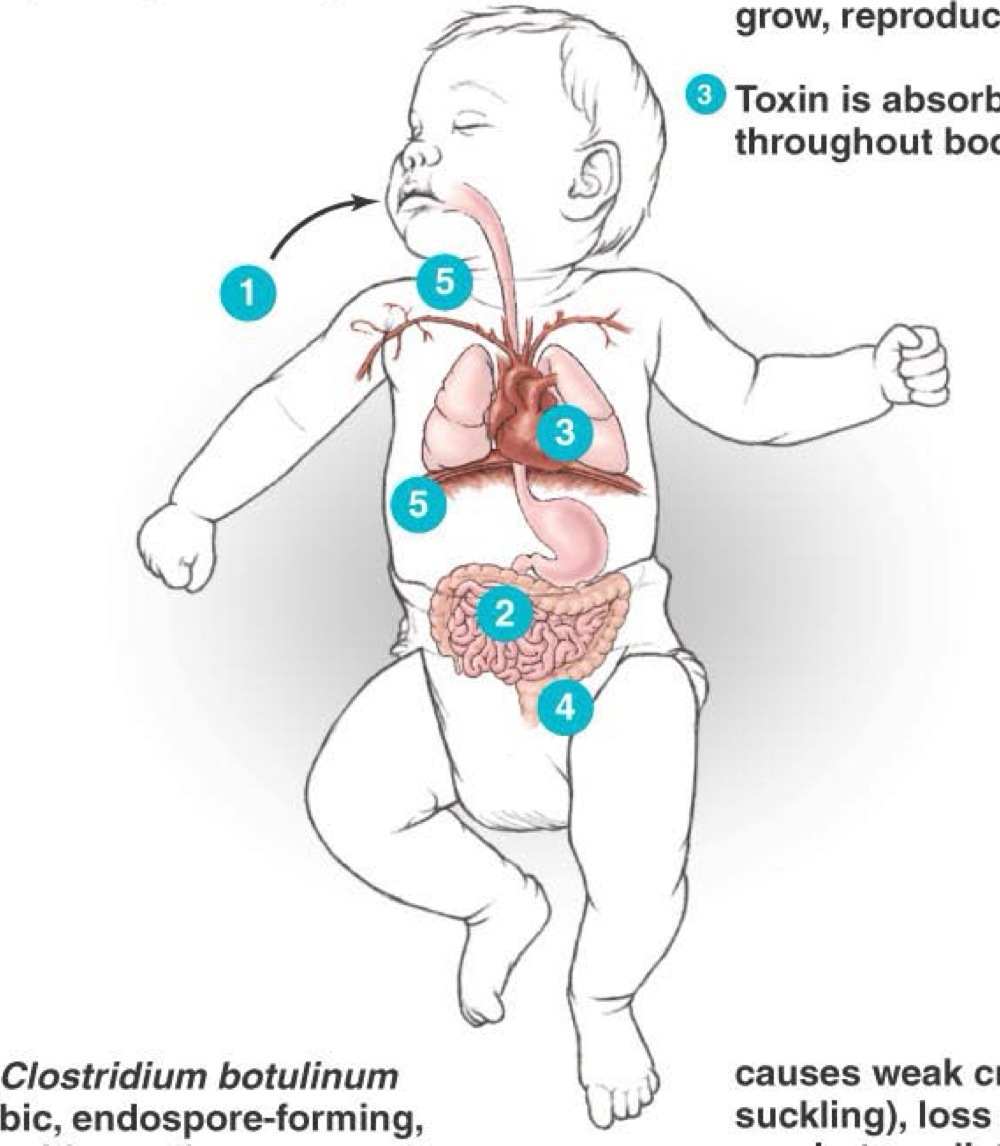
What occurs inside a baby when Clostridium botulinum spores are consumed?
Baby inhales or ingests C. botulinum endospores, particularly in honey
Endospore germinates in an aerobic environment of the intestinal tract. Vegetative cells grow, reproduce, and release botulinum toxin
Toxin is absorbed into the blood and circulates throughout the body
Botulism toxin produces constipation and a weak cry
Gradually, the toxin paralyzes muscles, including neck muscles and the diaphragm
Results in “floppy baby syndrome”
Clostridium tetani
Release a potent neurotoxin (tetanospasmin) when they die.
Morphology
Terminal endospore “Lollipop” appearance
Symptoms
Initial symptoms
Tightening of jaw and neck muscles, called “lockjaw”
Sweating, drooling, grouchiness, back spasm
Later symptoms
Affect involuntary muscle
Irregular heartbeating, blood pressure fluctuation, and extensive sweating
Prevention involves destroying endospores in contaminated food through proper canning techniques
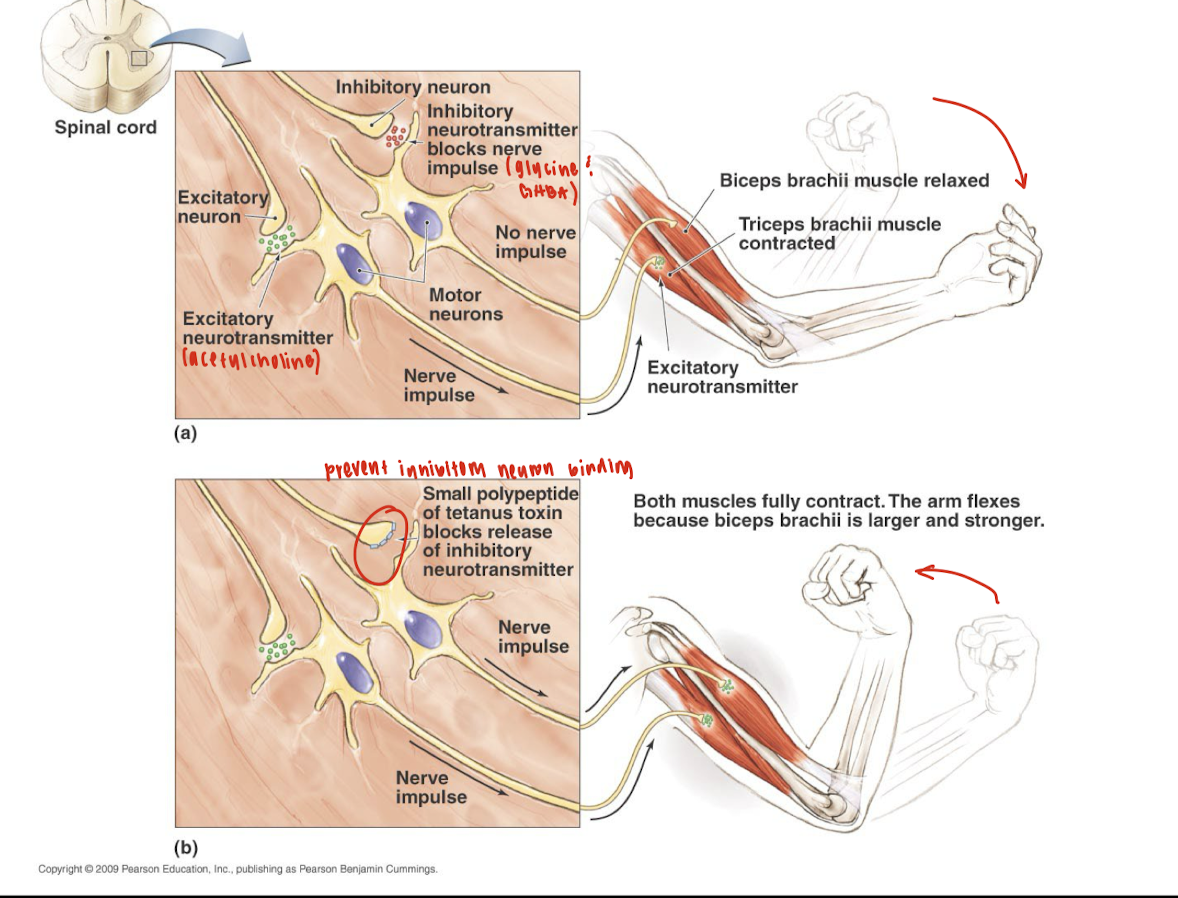
How does tetanus toxin interfere with normal neuronal signaling at the spinal cord, and what is the physiological consequence of this interference on muscle activity?
Tetanus toxin interferes with normal neuronal signaling by blocking the release of inhibitory neurotransmitters (such as glycine and GABA) from inhibitory interneurons in the spinal cord.
Normally, inhibitory neurons prevent excessive muscle contraction by inhibiting motor neurons. When tetanus toxin blocks this inhibition:
Inhibitory control is lost
Motor neurons remain overactive
This causes simultaneous contraction of opposing muscles (e.g., both biceps and triceps)
Leading to muscle rigidity and spasms, a hallmark of tetanus.
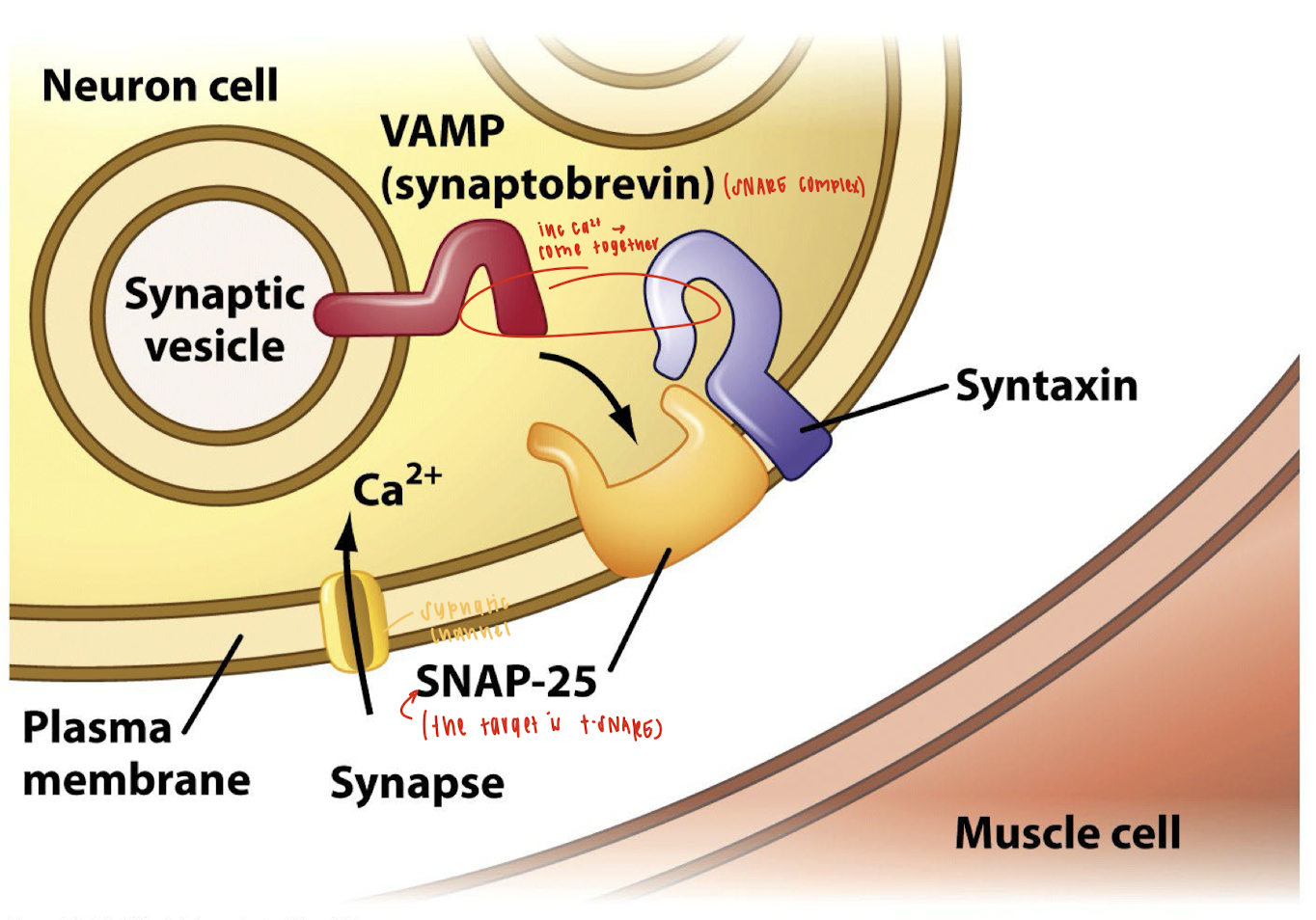
How does the mechanism of botulinum toxin inhibit neurotransmitter release at the neuromuscular junction?
The botulinum toxin inhibits neurotransmitter release by disrupting the SNARE complex, a group of proteins required for synaptic vesicle fusion with the presynaptic membrane. Here's how:
Endocytosis and Activation:
The toxin binds to the neuron cell membrane and enters via endocytosis.
Inside the endosome, the low pH breaks disulfide bonds in the toxin, activating it by allowing the proteolytic light chain to enter the cytoplasm.
Target Cleavage:
The active light chain cleaves SNARE proteins, which are essential for vesicle fusion.
Specifically, botulinum toxin cleaves synaptobrevin (VAMP), a key vesicle-associated SNARE protein.
Effect:
Without VAMP, the synaptic vesicle cannot fuse with the plasma membrane.
As a result, acetylcholine cannot be released into the synaptic cleft, and muscle contraction is blocked, causing flaccid paralysis.
What is the overall structure of tetanus and botulinum toxins?
They are single polypeptides (similar to diphtheria toxin) consisting of a heavy chain and a light chain, connected via a disulfide bond.
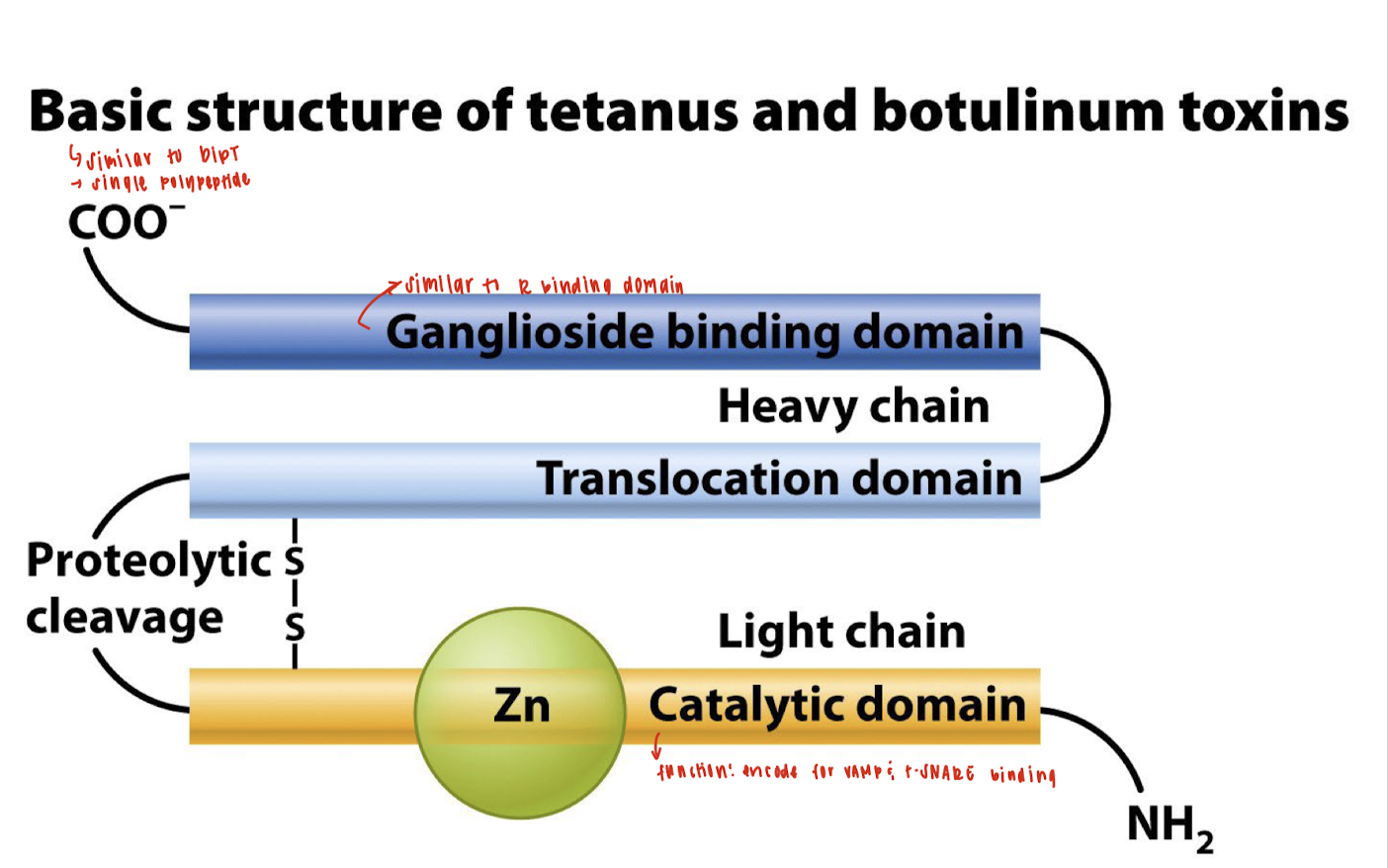
What are the main domains of the heavy chain in tetanus and botulinum toxins?
Ganglioside Binding Domain – for neuronal targeting (similar to receptor-binding domains).
Translocation Domain – helps transport the light chain into the host cell cytoplasm.
What is the role of the ganglioside binding domain?
It binds to neuronal gangliosides, allowing toxin entry into nerve cells.