Lab Final Experiments 7-10
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Write a balanced chemical equation for the Fischer esterification reaction between i-pentyl alcohol and acetic acid.

Calculate the volume (mL) of water formed from full esterification of 1 g of acetic acid (in the above reaction) assuming the reaction is 100% complete.
18/60.05=0.299
Explain how the IR spectra can distinguish between i-pentyl alcohol and acetic acid.
OH has a broad peak 3200-3600.
C=O in acetic acid 1680-1750.
C-O in pentyl 1000-1300.
Draw the structure of the ester that can be prepared from 2-propanol and acetic acid. Give both the common and IUPAC names of the ester product.
Common: isopropyl acetate
IUPAC: isopropyl ethanoate
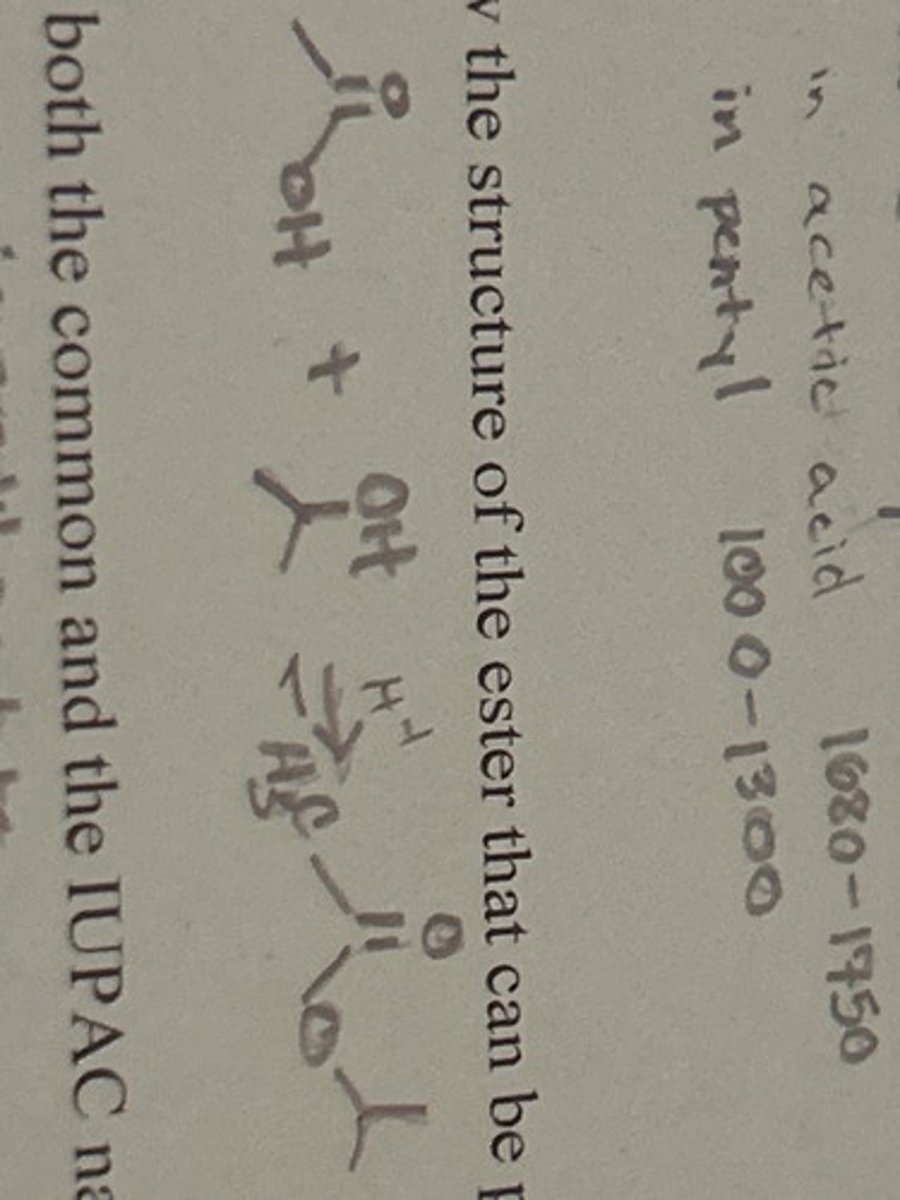
Esters can undergo hydrolysis under acidic conditions to form carboxylic acids and alcohols. Write a chemical equation for the hydrolysis of ethyl acetate.

Explain the purpose of washing the organic layer containing the ester with 10% aqueous NaHCO3.
It removes unreacted benzoic acid.
Write a detailed mechanism of the acid-catalyzed esterification reaction between i-pentyl alcohol & acetic acid.

Give the IUPAC name i-pentyl acetate.
3-methylbutyl acetate
Write a mechanism for alkaline hydrolysis of methyl salicylate.

Write the chemical equation for acidic hydrolysis of aspirin.
C9H8O4 + H2O -> C7H6O3 + C2H4O2
What is the purpose of washing the soap product with cold water?
It helps to remove impurities.
What do magnesium % calcium salts diminish the cleansing effectiveness of soap?
They harden water.
Explain why hard water does not affect the cleansing effectiveness of synthetic detergents.
It is made from synthetic resources and is not natural.
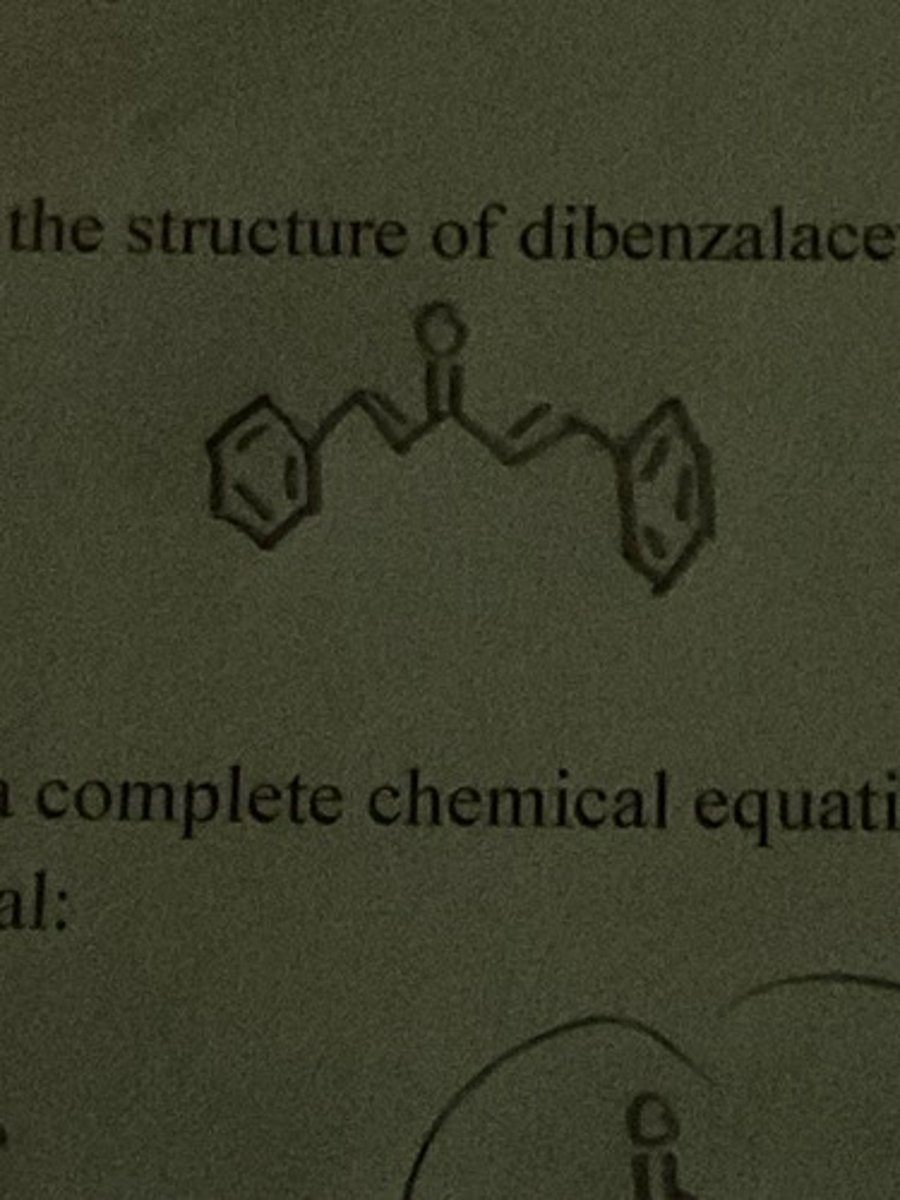
Write the structure of monobenzalacetone.
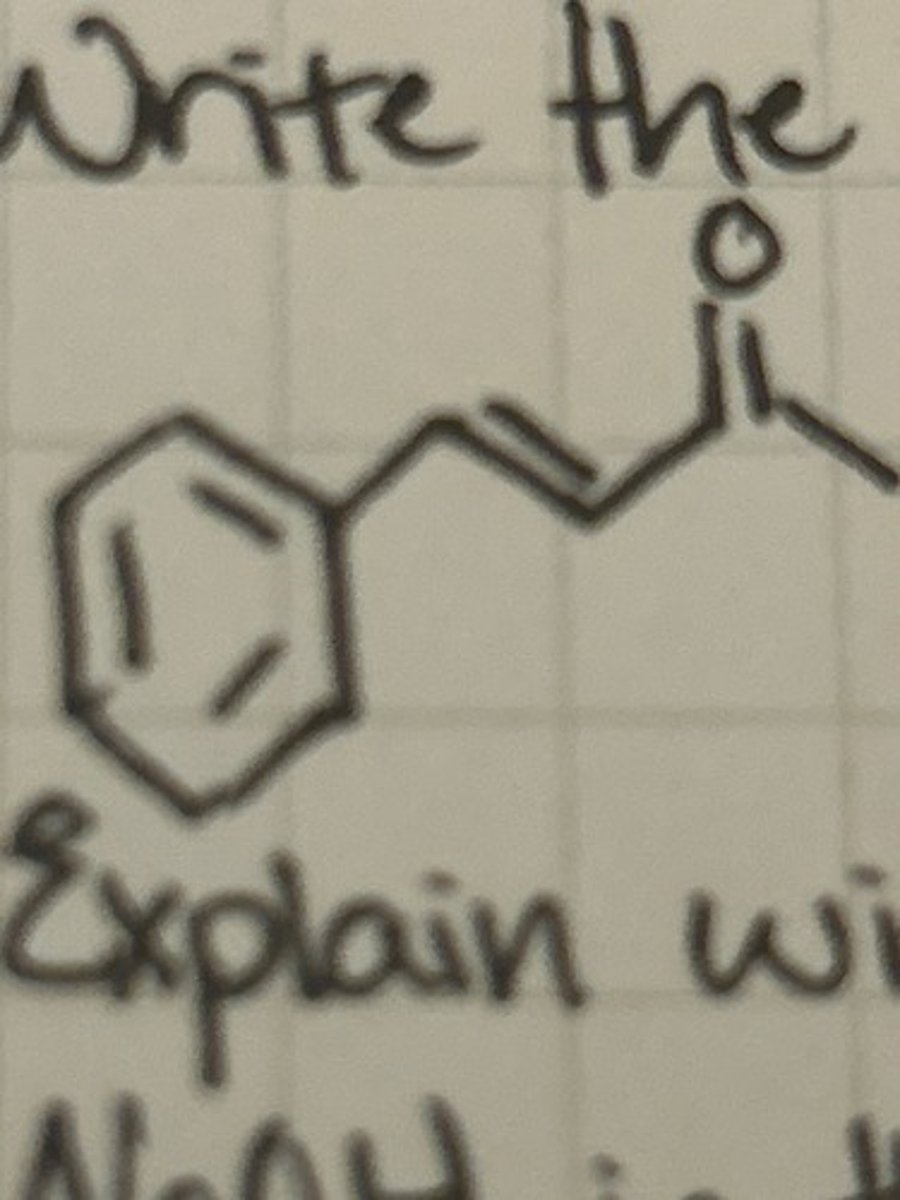
Explain with a chemical equation the role of NaOH in the synthesis of dibenzalacetone.
NaOH will give OH- ion which will initiate deprotonation of acetone thereby initiating the reaction.

What is the purpose of washing the product with cold water?
To remove the NaOH impurities.
Write the structure of one possible organic side-product during dibenzalacetone synthesis.
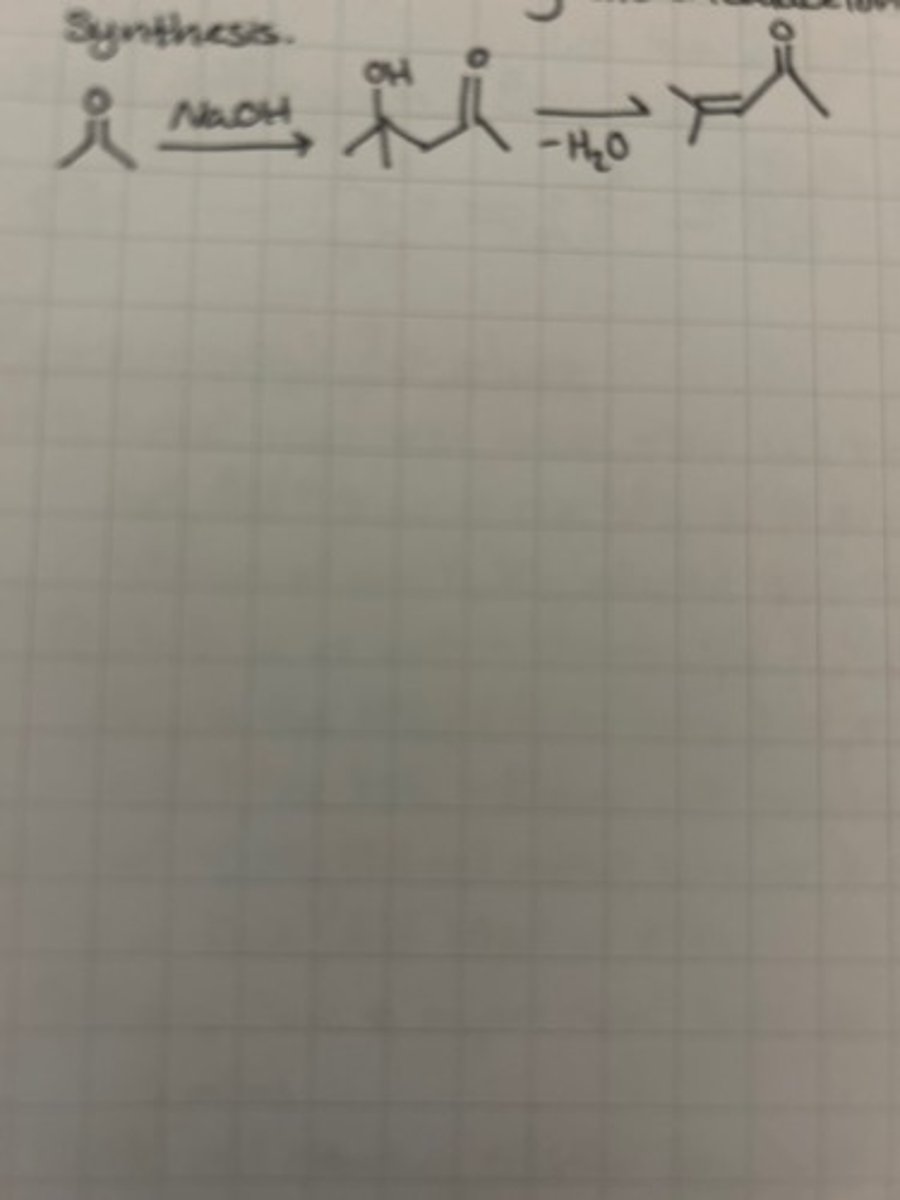
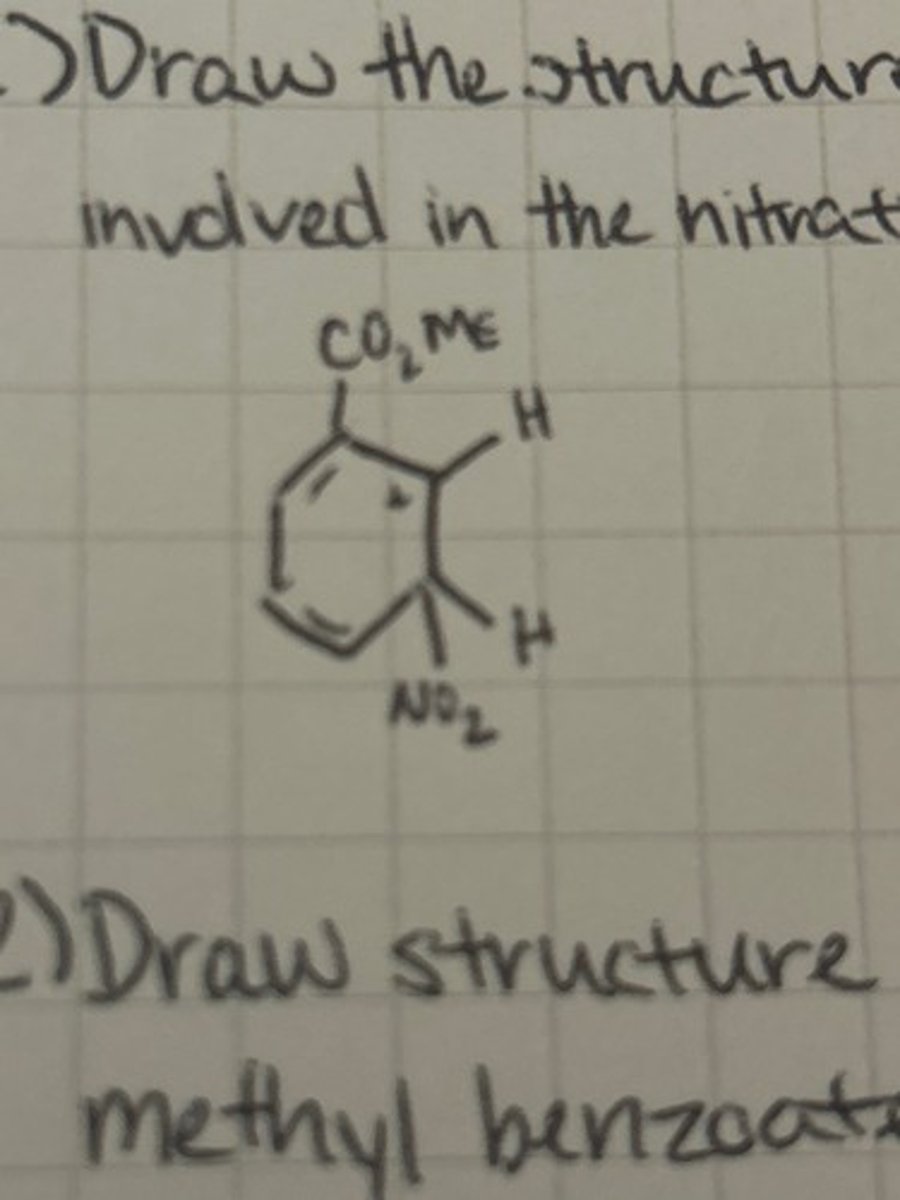
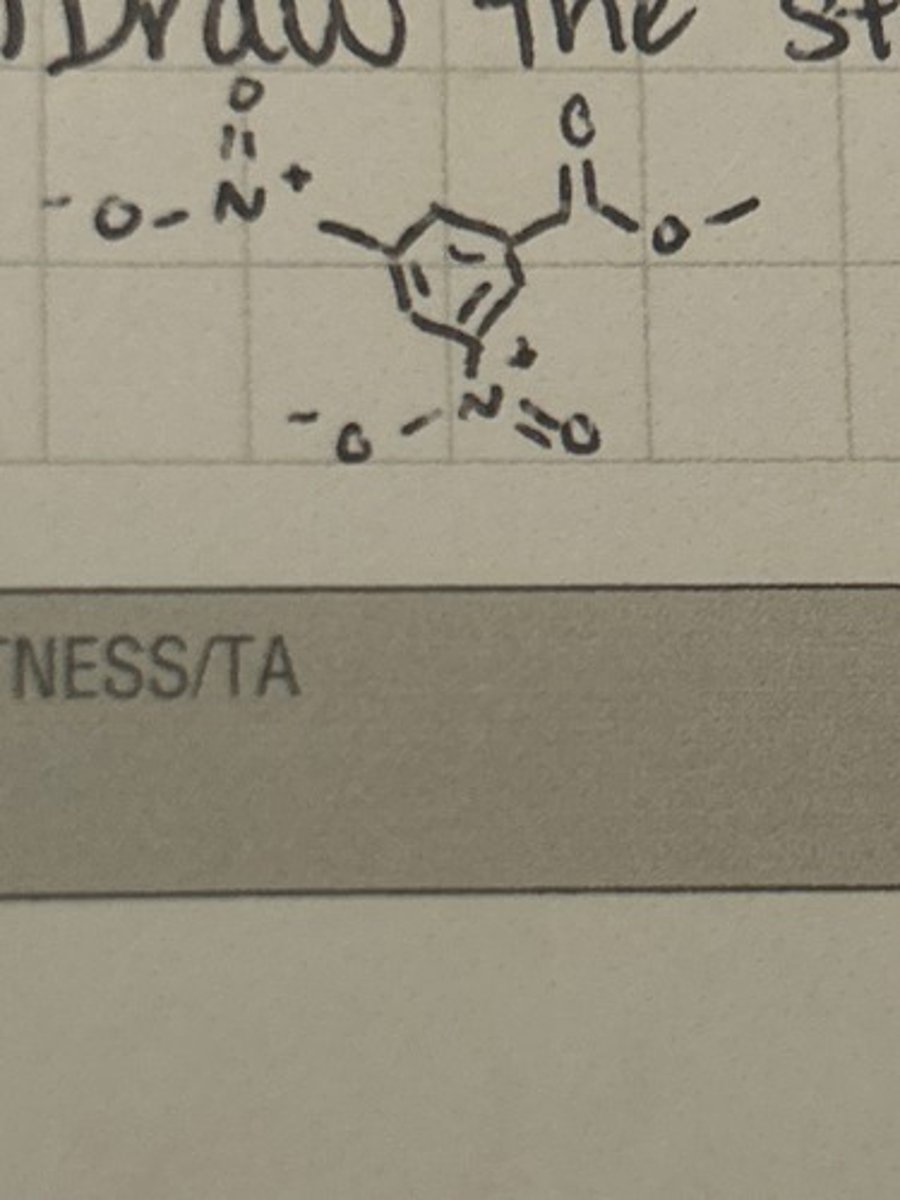
Draw the structure of the arenium ion intermediate involved in the nitration of methyl benzoate.
Draw the structure of the nitration product of methyl benzoate.
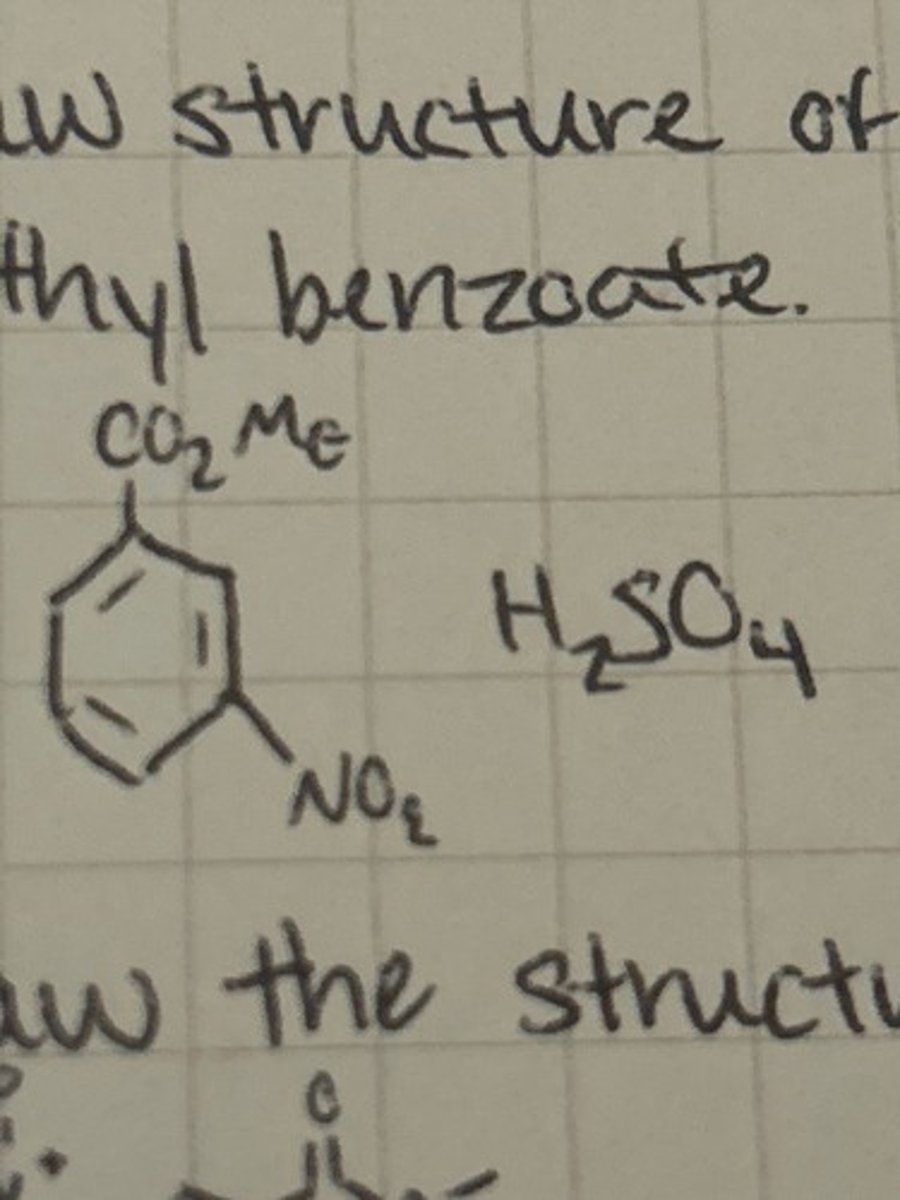
Draw the structure of methyl 3,5 dinitrobenzoate.
Which of the following aromatic compounds undergoes nitration faster than benzene does? Explain.
Ph-CH3, Ph-Cl, Ph-OH, Ph-Br, Ph-NO2, Ph-Ph
Ph-CH3: CH3 has an electron donating effect
Ph-OH: -OH group donates electrons to the ring with resonance, increasing nitration rate
Ph-Ph: Ph activates benzene ring by electron donation
In the synthesis of explosive trinitrotoluene (TNT) toluene is nitrated three times. Explain why the first nitration step proceeds much faster than the second & the third nitration steps.
Nitration causes ring activation. NO2 is electron withdrawing & decreases electron density on the benzene ring. Thus the next NO2 attack will not be as spontaneous.
Explain the health hazards of ingesting industrial ethanol.
nausea, vomiting, itching, burning, and irritation
Draw the structure of the triglyceride that can be formed from stearic acid CH3(CH2)16COOH

Give two examples of synthetic detergents. Give the name of the active surfactant in each.
Sodium lauryl sulfate
Liquid dishwashing detergent
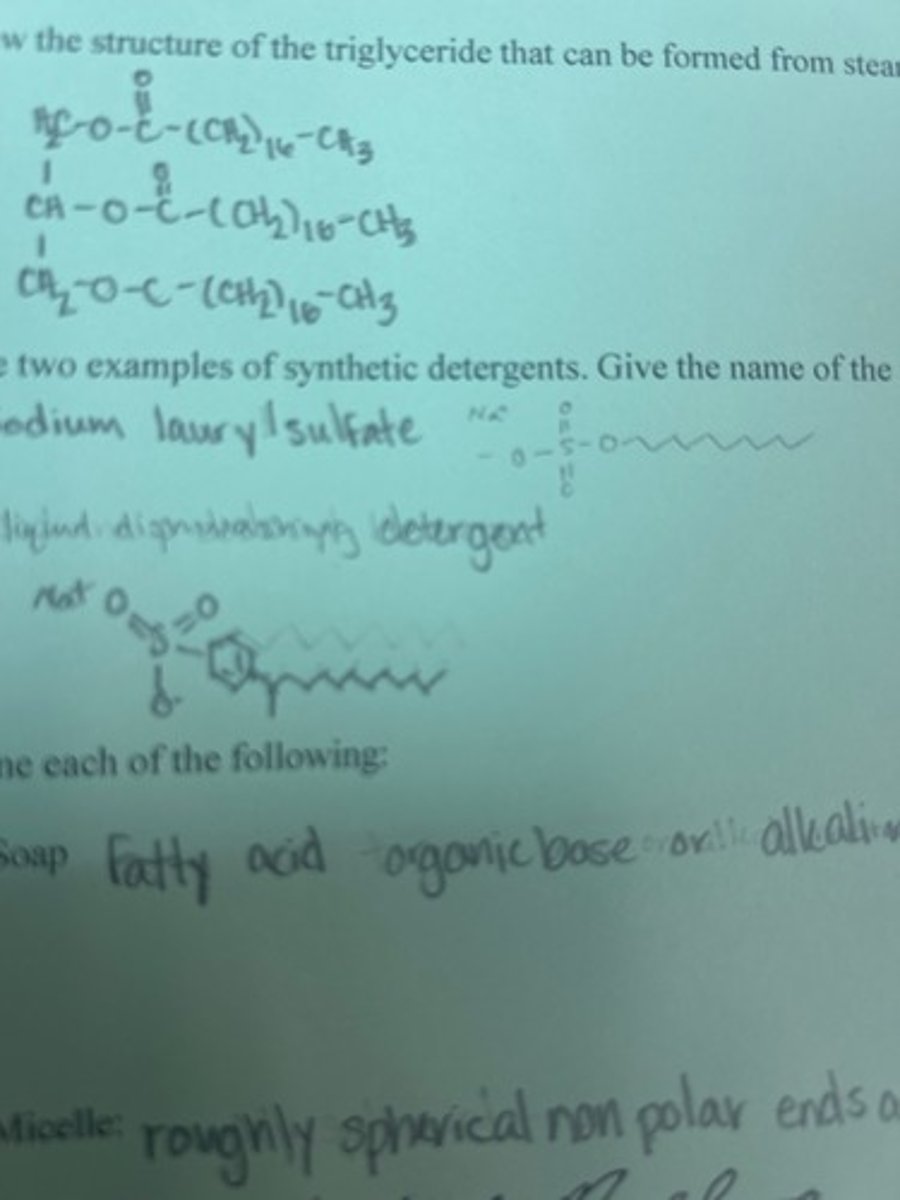
What is soap?
fatty acid organic base or alkaimetal
what is micelle
minute water-soluble droplets of soap molecules with the hydrophilic carboxylate ions encasing the hydrophobic non-polar chains
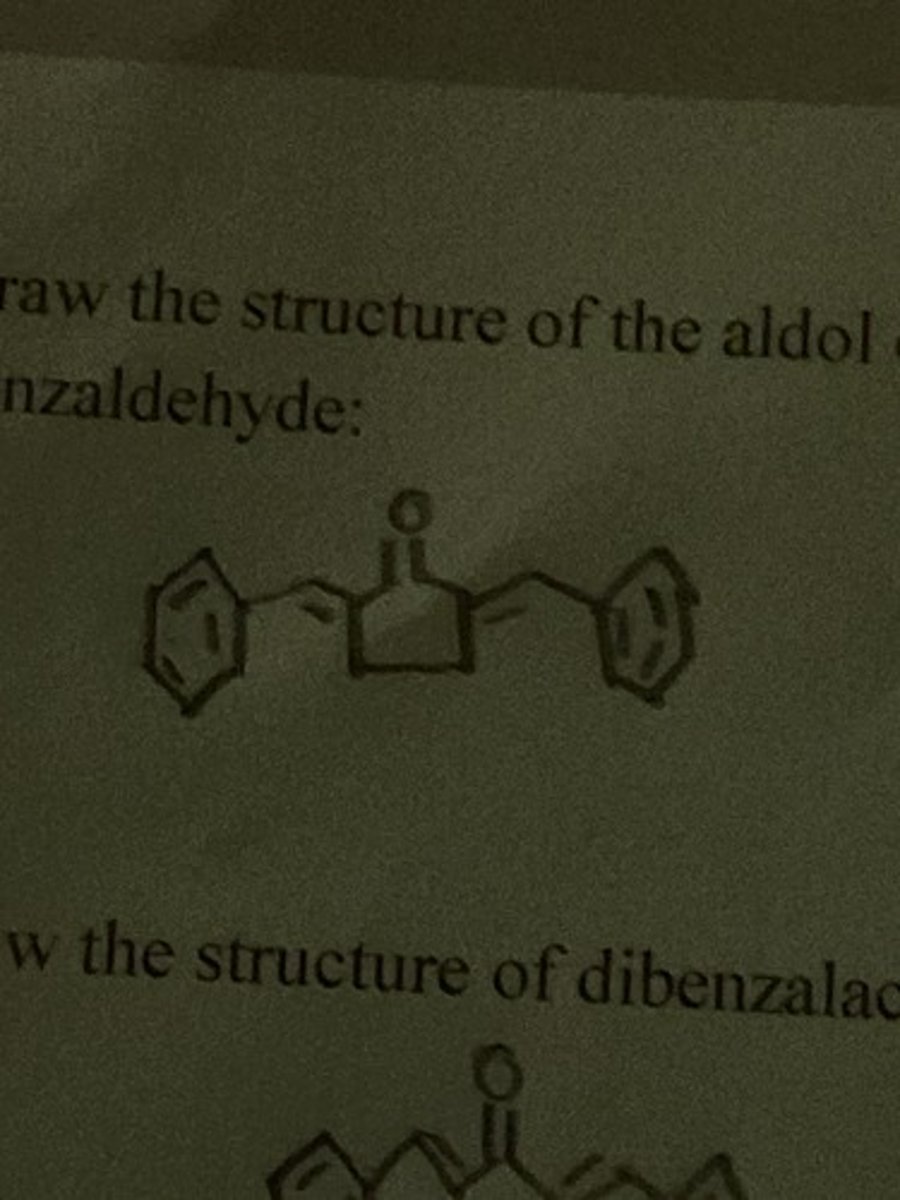
Draw the structure of the aldol condensation between cyclopentanone and two equivalents of benzaldehyde
Draw the structure of dibenzalacetone.
Write a complete chemical equation of base-catalyzed aldol condensation involving two molecules of propanol.
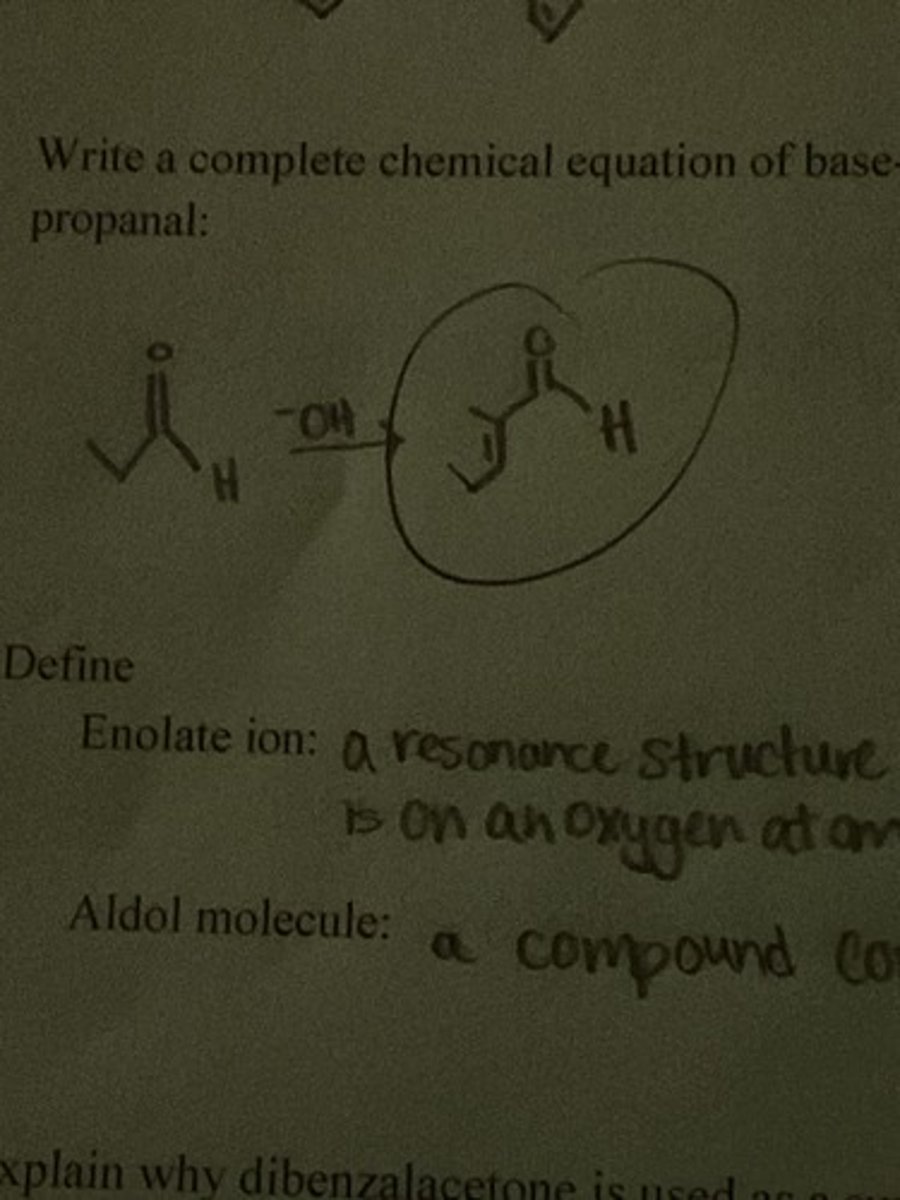
Define an enolate ion.
a resonance structure of the carbanion where the negative charge is on an oxygen atom
aldol molecule
a compound containing an aldehyde and alcohol group
Explain why dibenzalacetone is used as sunscreen
it effectively blocks uv radiation because of the extremly conjugated C=C bonds
What is the safety hazard of each of concentrated sulfuric and nitric acids?
it is corrosive and can irritate/burn skin and clothes
Which acid is stronger, sulfuric acid or nitric acid? Explain
Sulfuric because it is diprotic and donates two protons while nitric is monoprotic and only donates one
Explain with a chemical equation, why methyl benzoate dissolves in conc. H2SO4 but not in water.
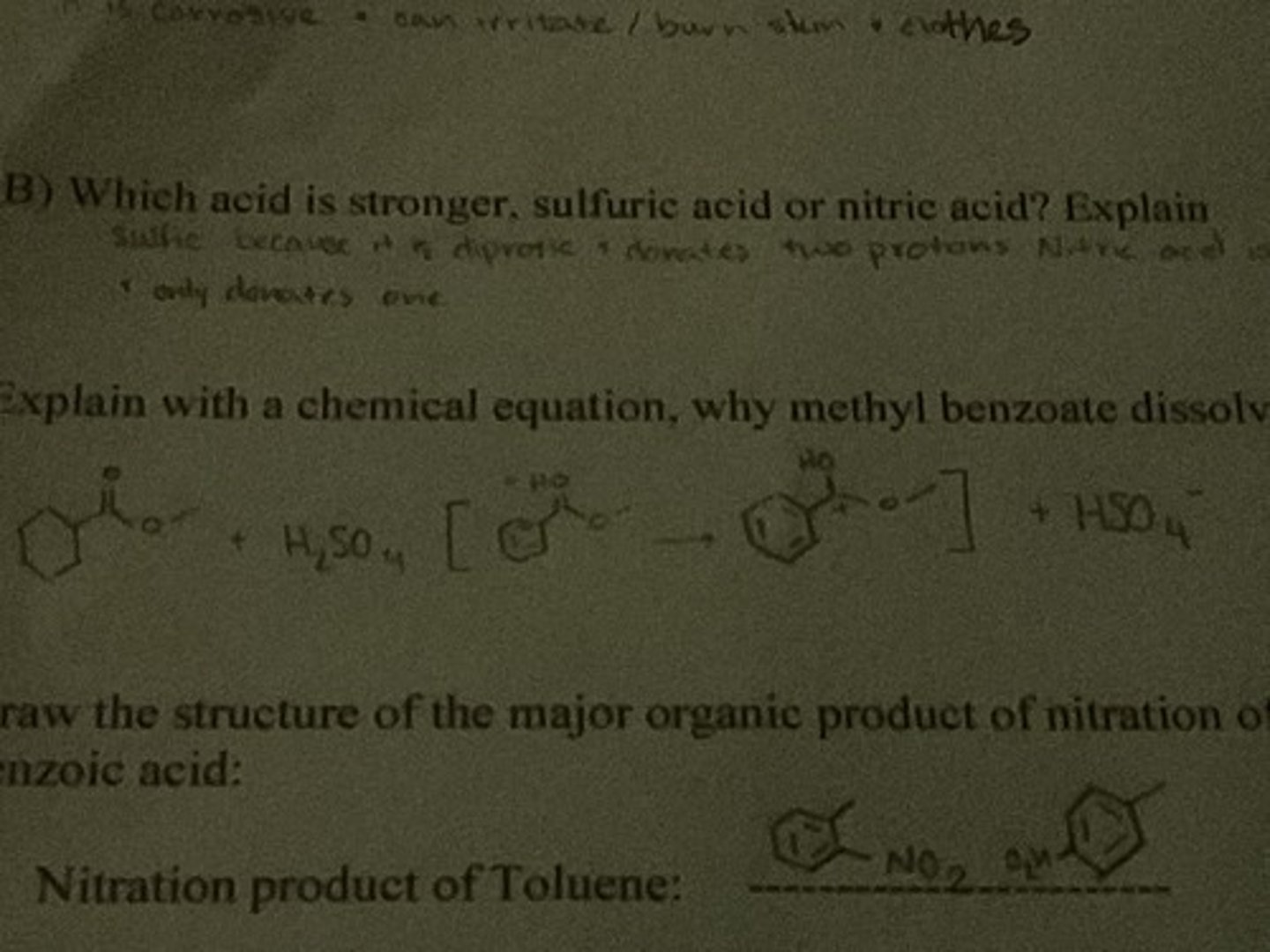
Draw the structure of the major organic product of nitration of Toluene.
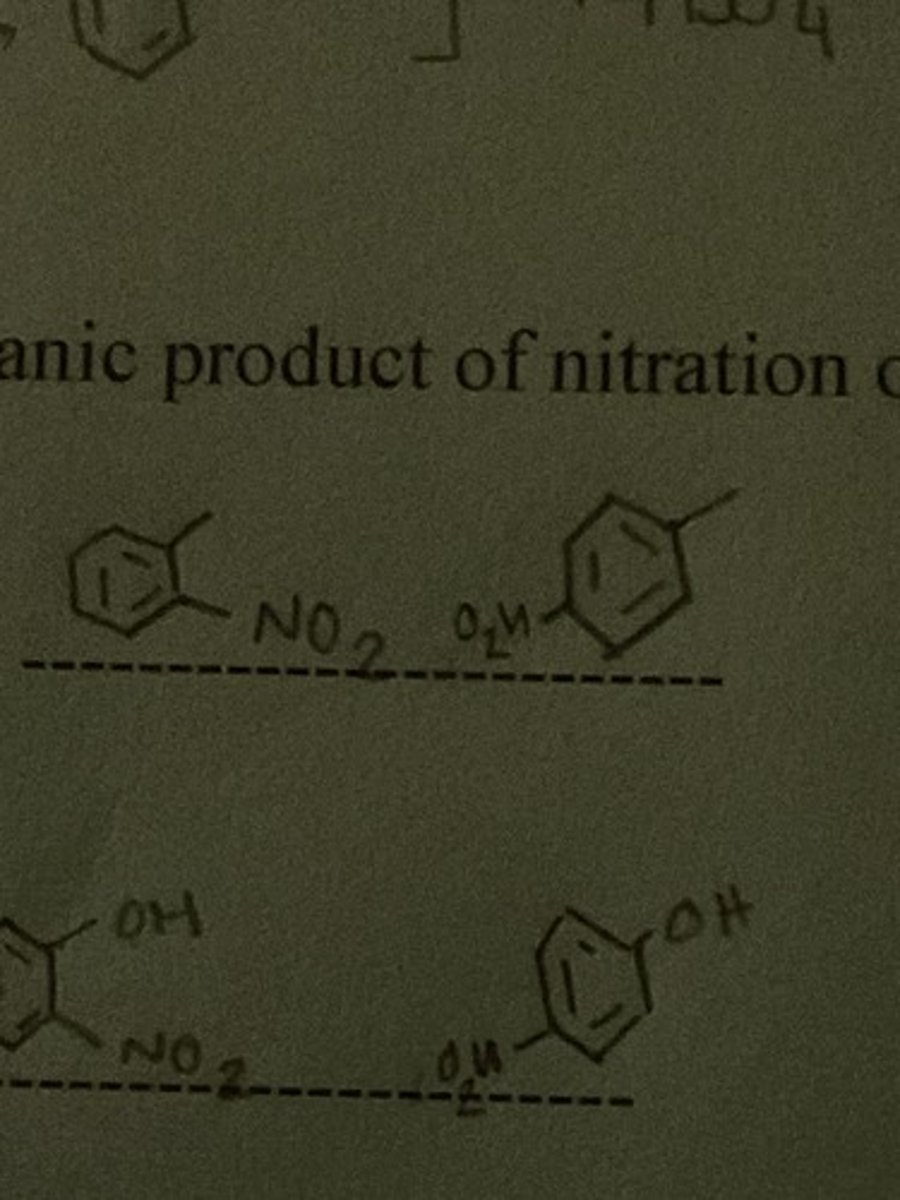
Draw the structure of the major organic product of nitration of phenol.
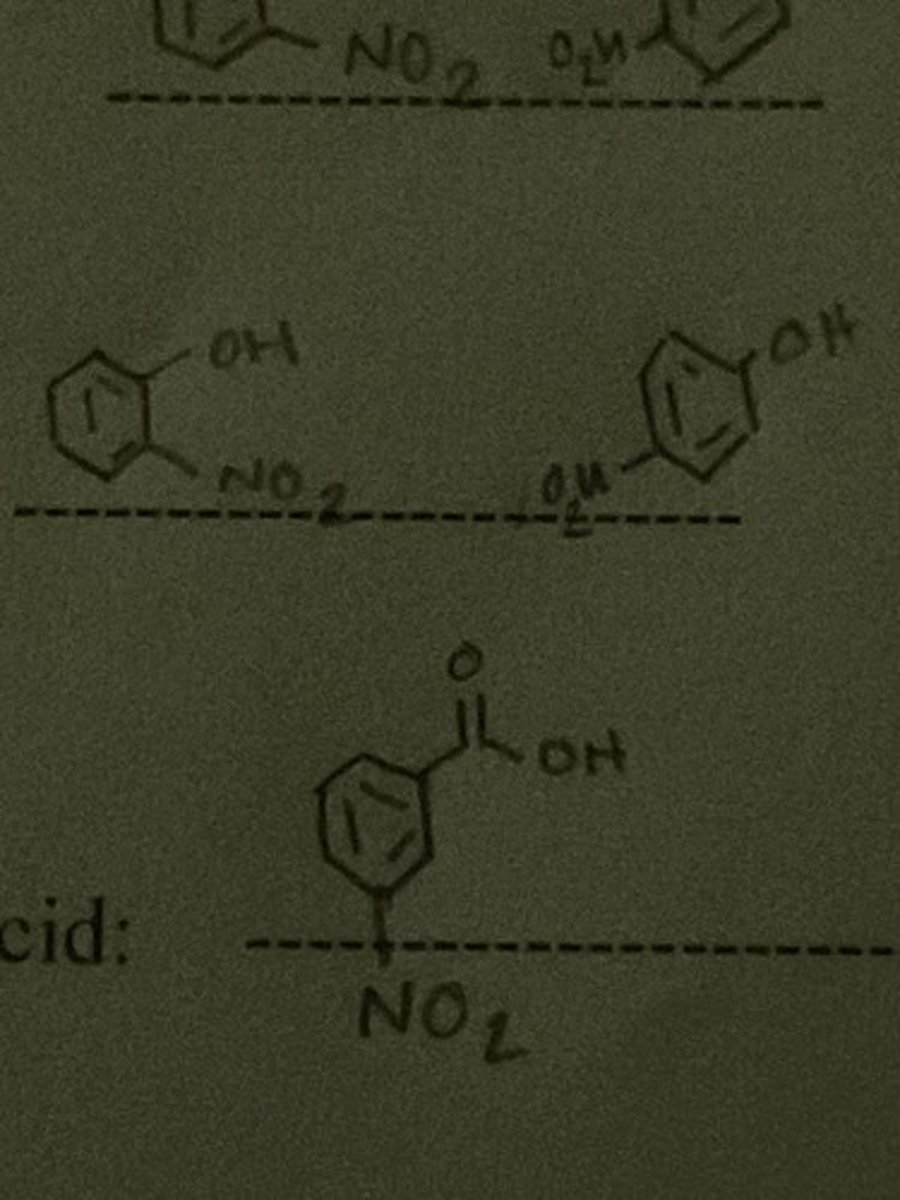
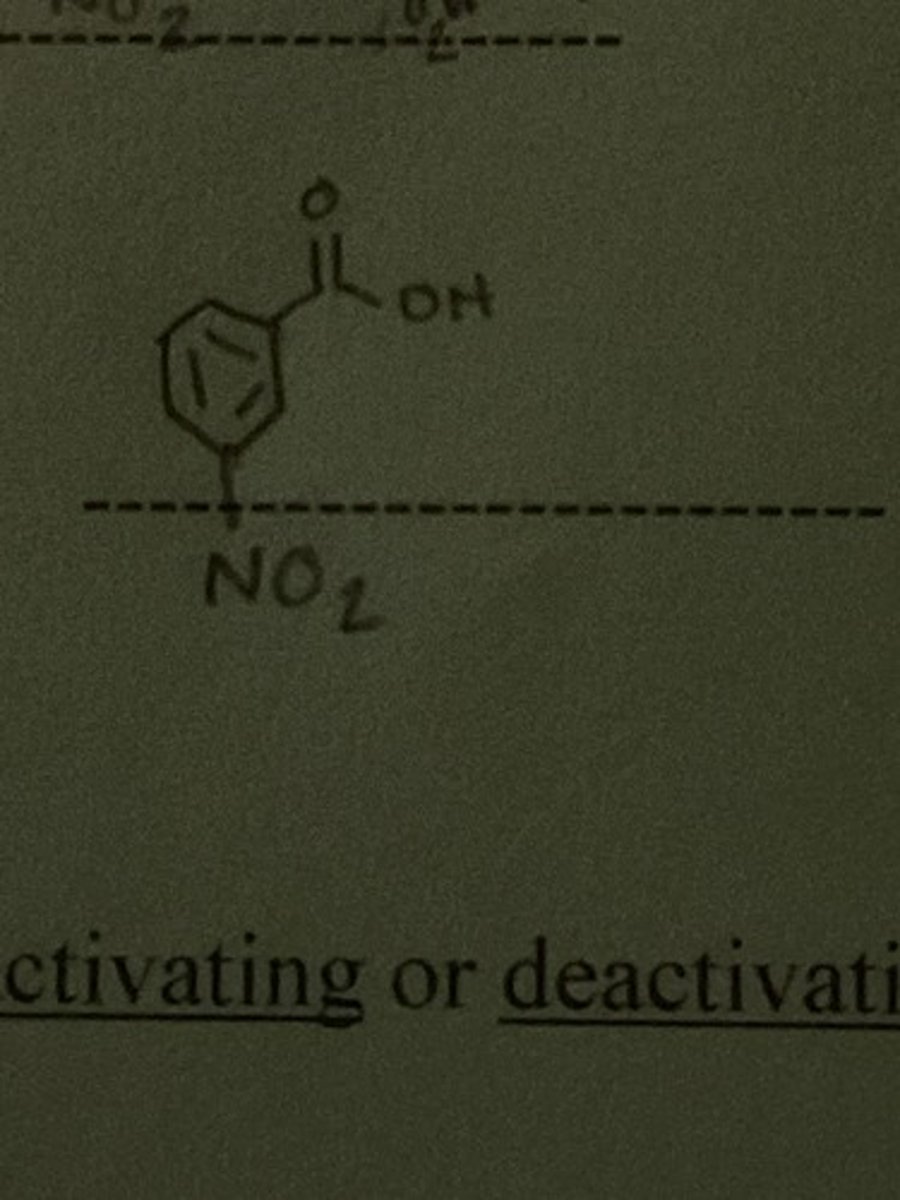
Draw the structure of the major organic product of nitration of benzoic acid
Is CH3 deactivating or activating?
activating
Is NO2 deactivating or activating?
deactivating
Is COOH deactivating or activating?
deactivating
Is OH deactivating or activating?
activating
Explain why it is important to conduct the nitration of methyl benzoate at low temperature.
There is low chance of denitration