Extraction lab
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
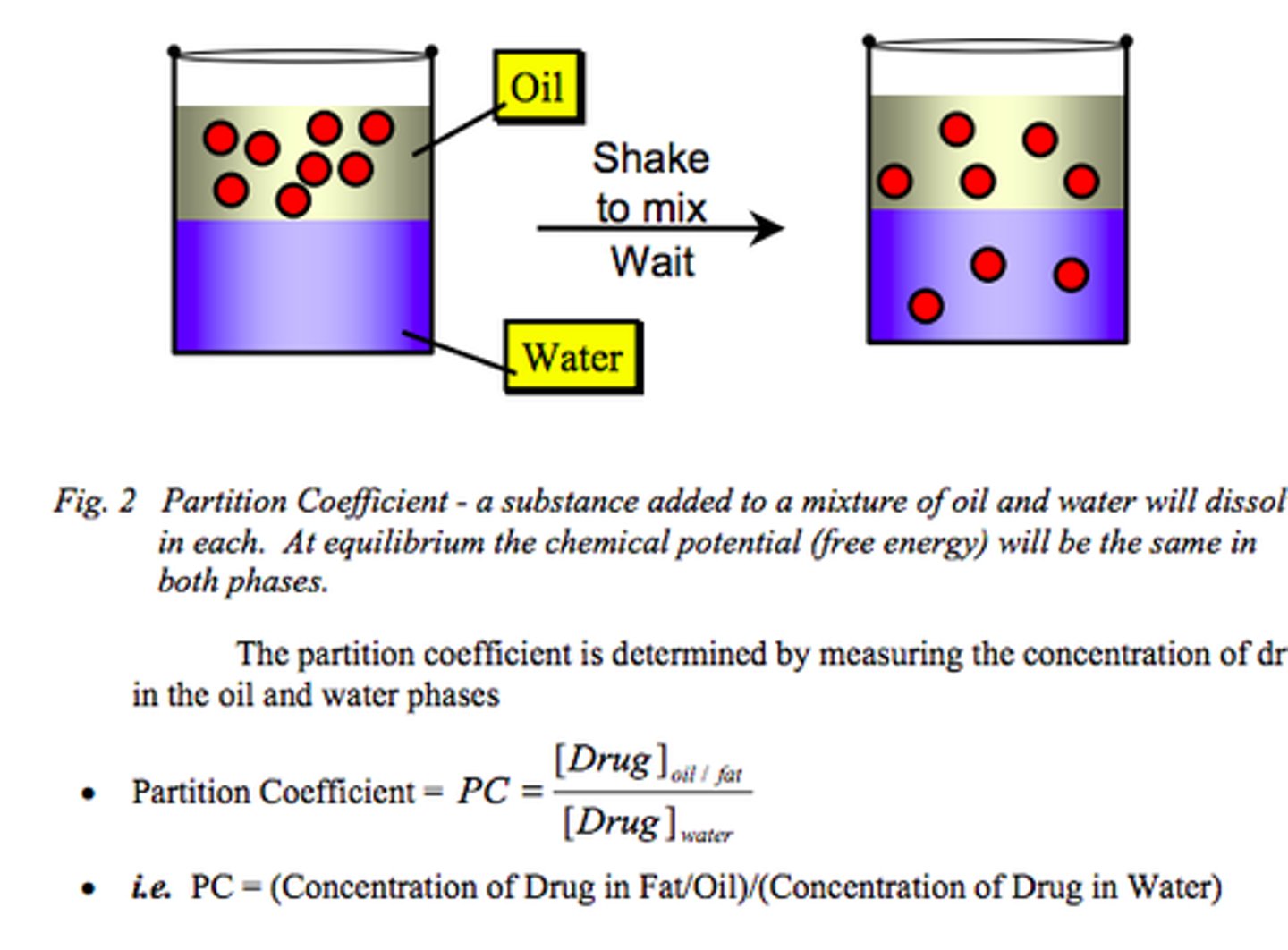
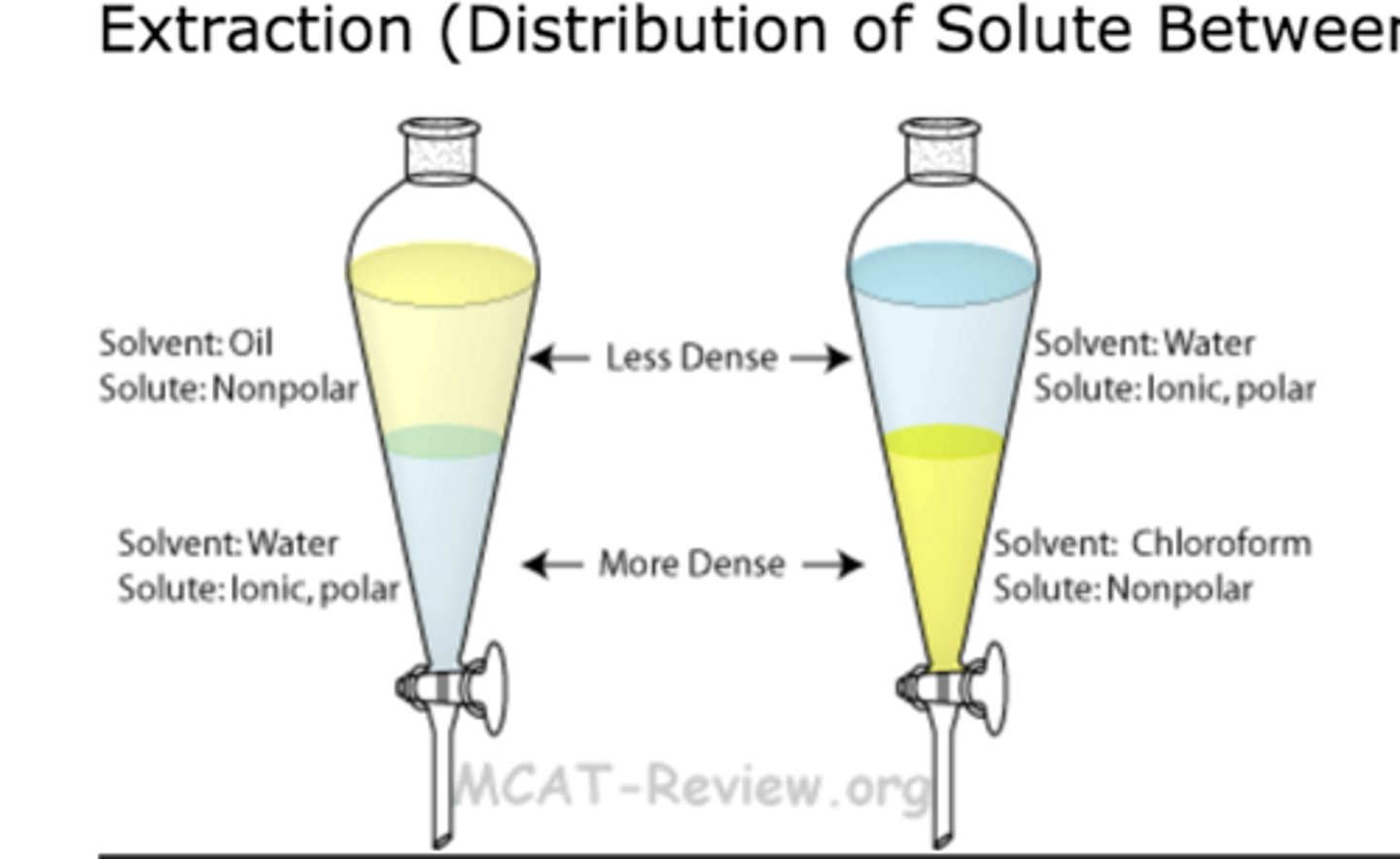
Solvent extraction
separates on the basis of solubility and solvent density
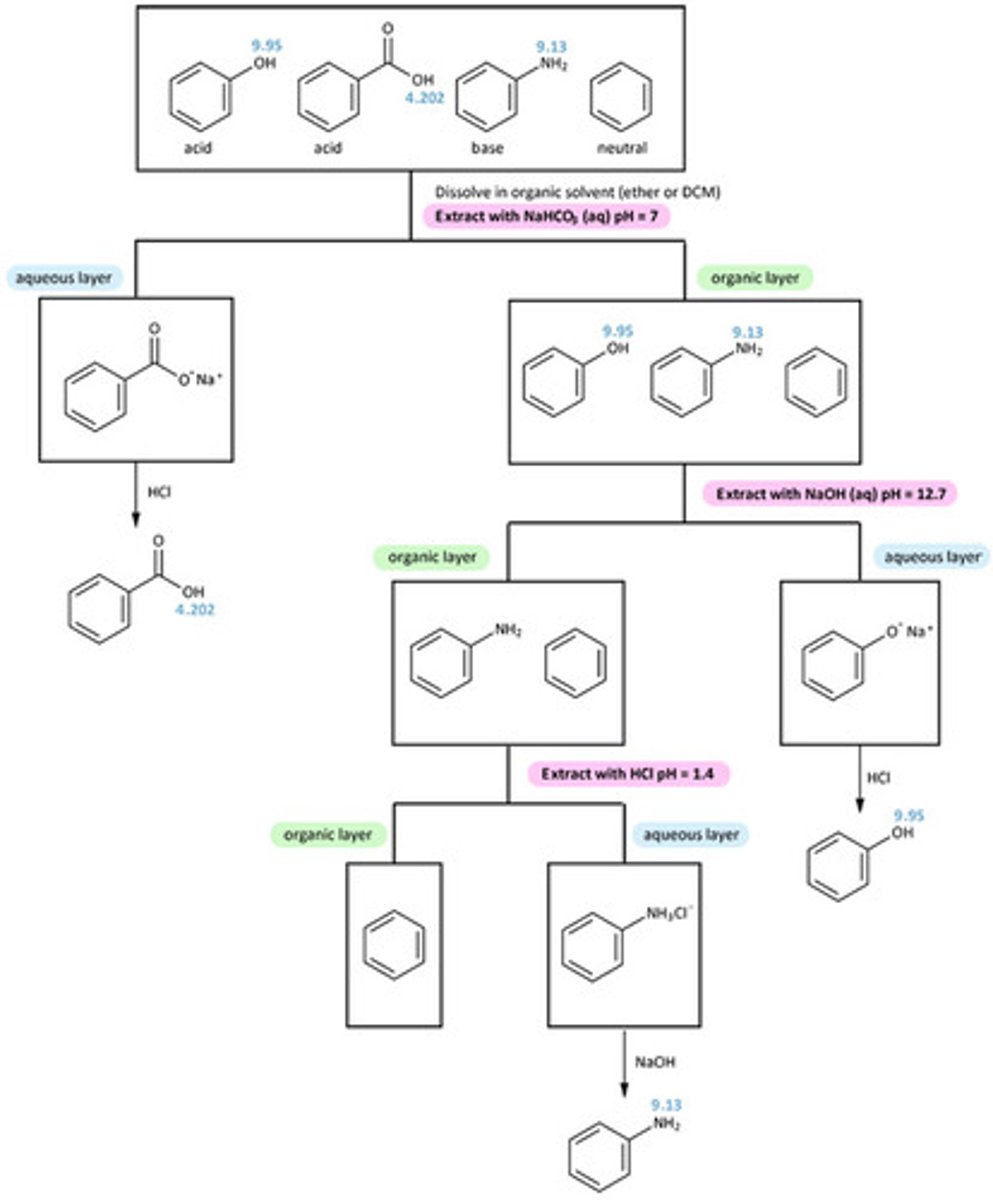
Acid-base extraction
Separates compounds based on acid-base properties.
neutral organic compounds
ketones, aldehydes, esters, anhydrides, and hydrocarbons
acidic organic compounds (strong to weak)
carboxylic acids, alcohols, and phenols
basic compounds
amines, some amides
strong bases
NaOH, KOH
strong acids
HCl
weak base
NH3, NaHCO3
acid
A substance that increases the hydrogen ion concentration of a solution.
base
A substance that decreases the hydrogen ion concentration in a solution.
neutral solvents
ether, DCM
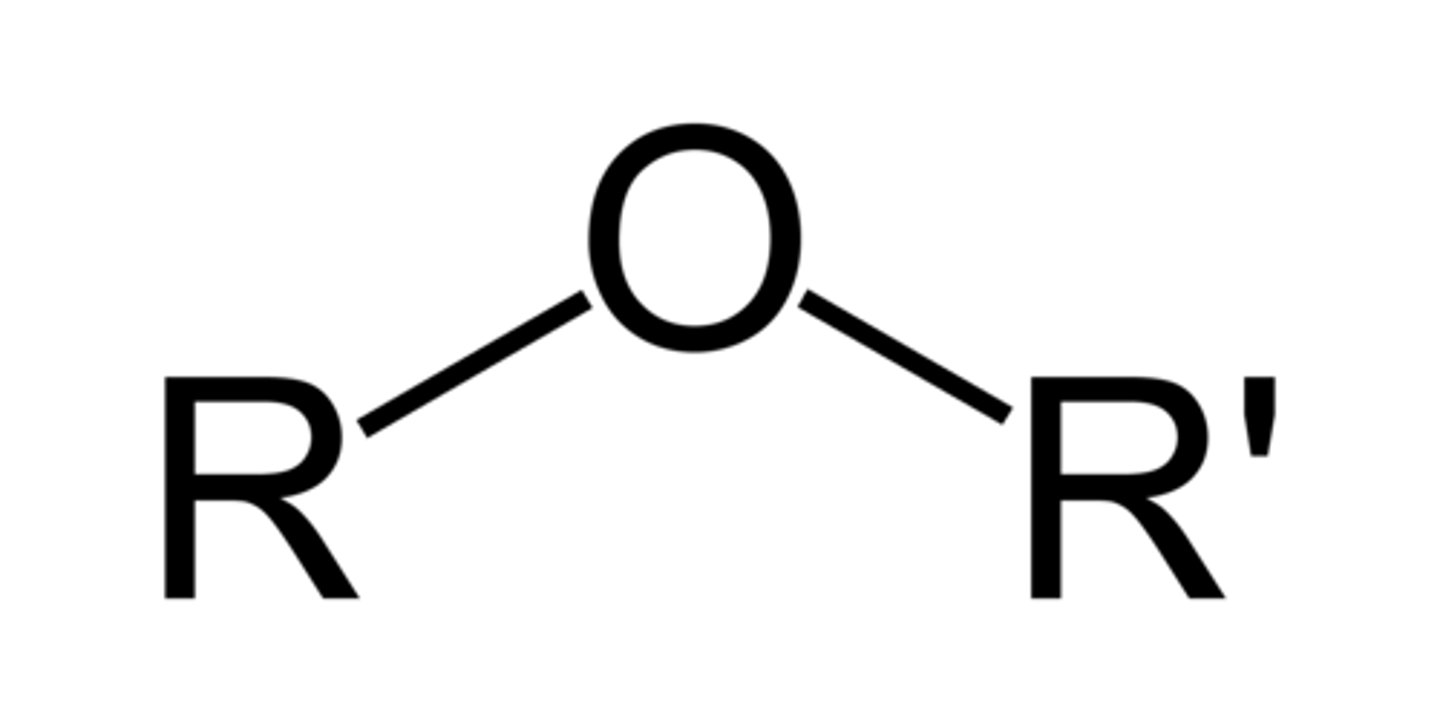
ether
R-O-R
DCM
Dichloromethane (CH2Cl2)
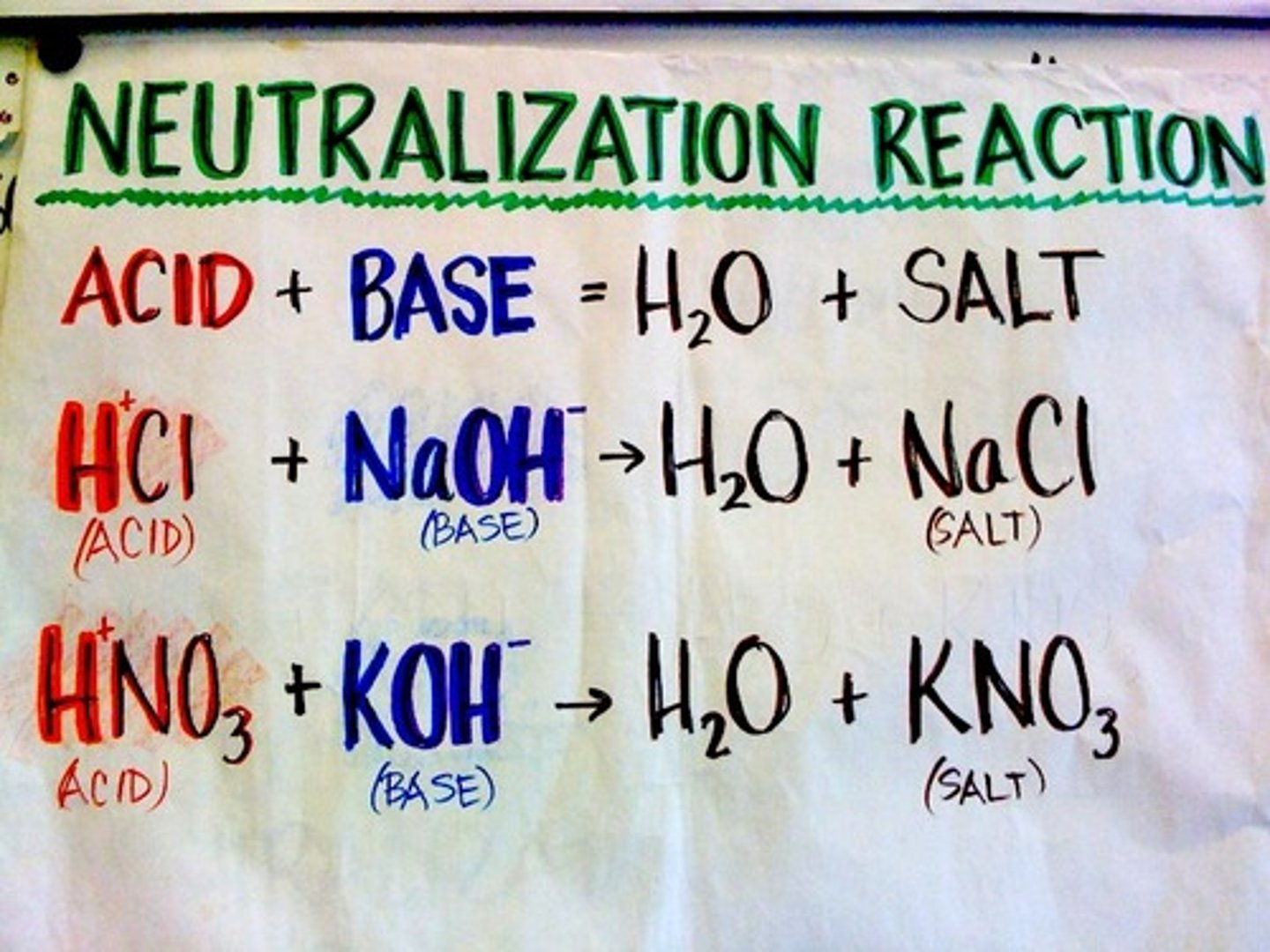
neutralization reaction
a reaction in which an acid and a base react in an aqueous solution to produce a salt and water
organic compounds denser than water
halogenated solvents
trichloromethane
Chloroform (CHCl3)
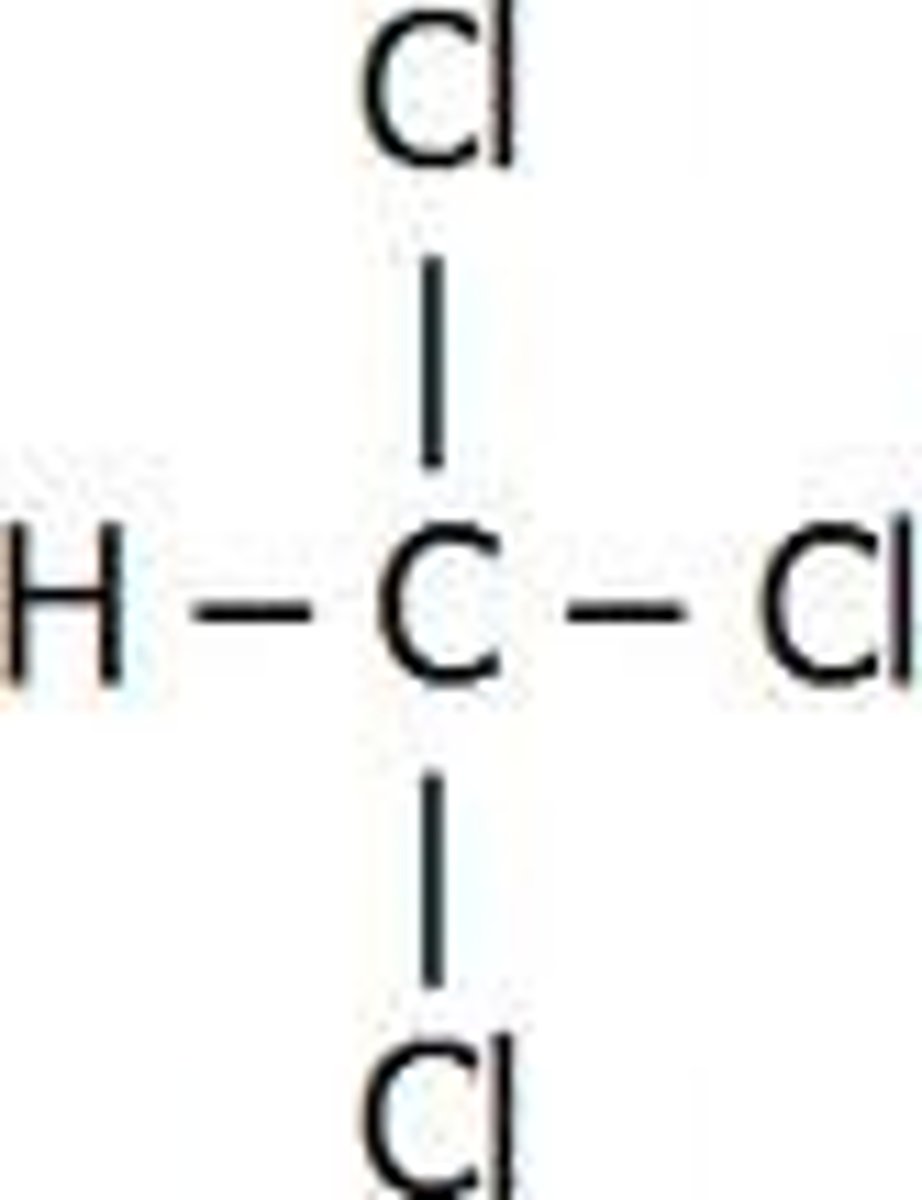
carbon tetrachloride
CCl4
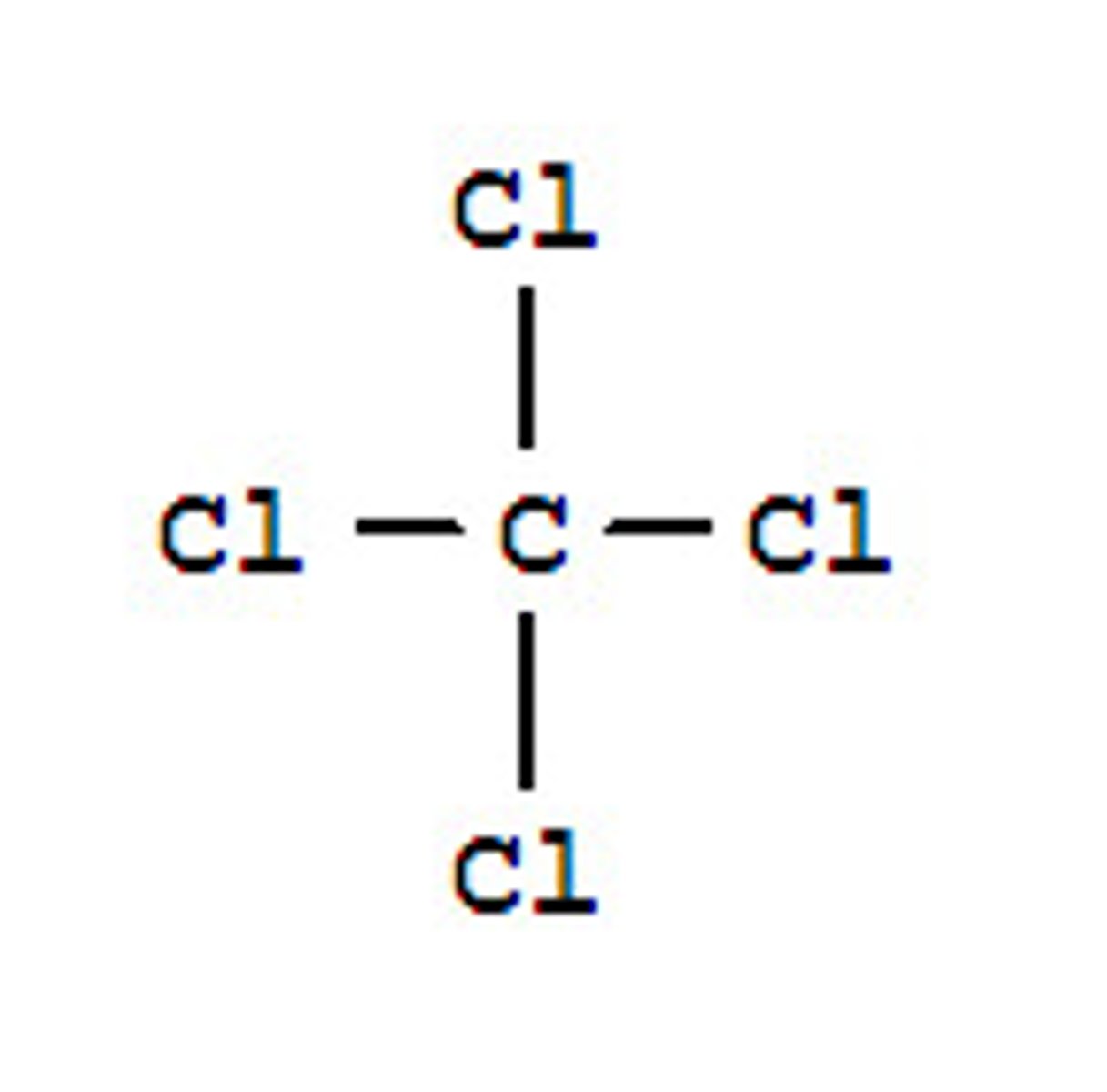
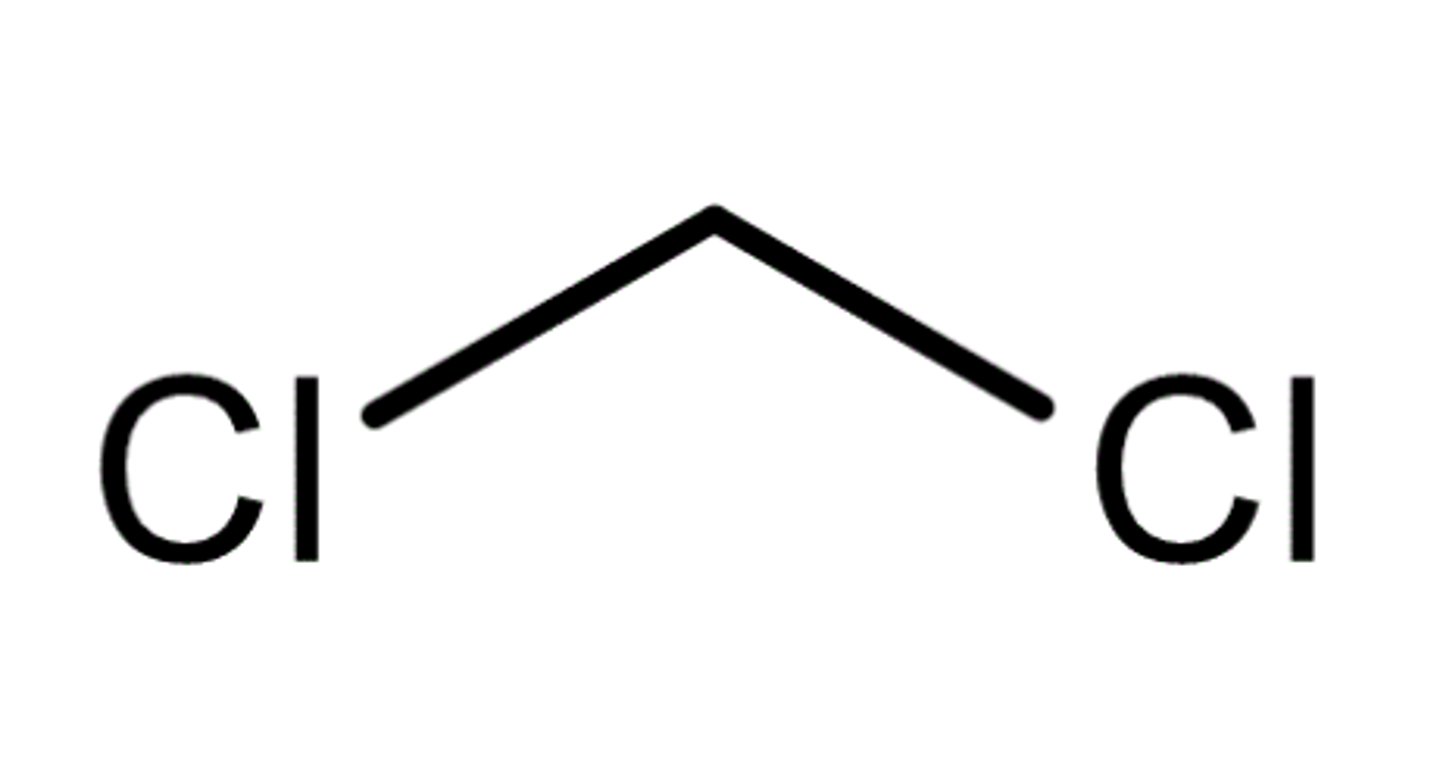
organic compounds lighter than water
ether, hexane, pentane
partition coefficient
The ratio of the solute concentrations in the organic and aqueous phase