BIO230 Lab 4: Live Cell Imaging Techniques
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
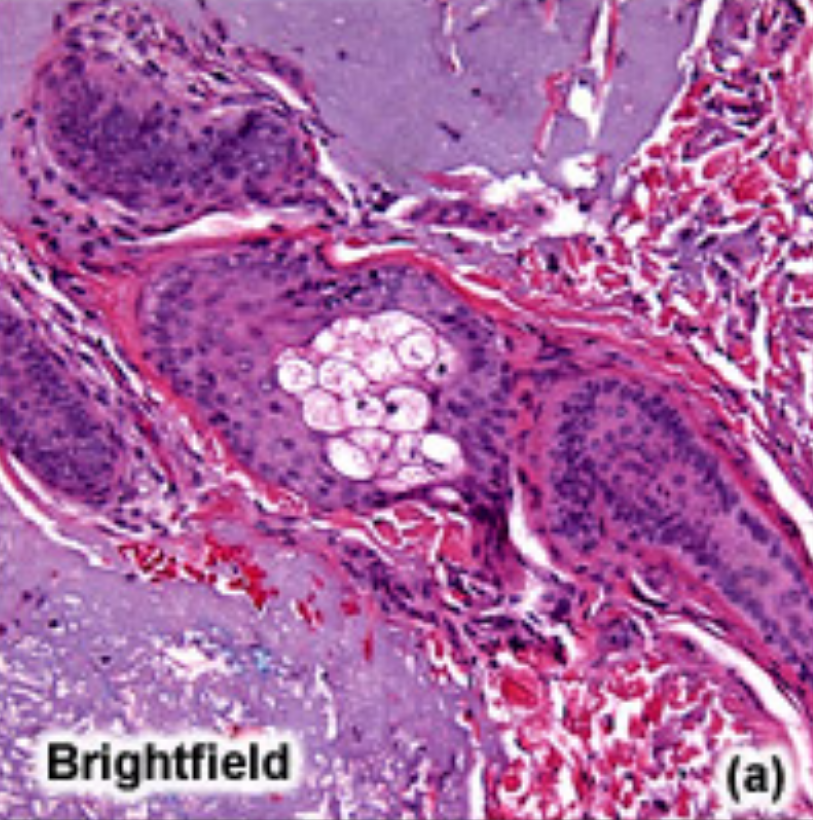
Brightfield microscopy
Standard direct light microscopy.
Capable of viewing living and fixed specimens by using visible light and optical lenses to magnify an image.
Staining or natural pigmentation can provide image contrast, whilst the Koehler illumination technique ensures uniform specimen lighting.
Also, can’t see internal mechanisms with this unless fixation
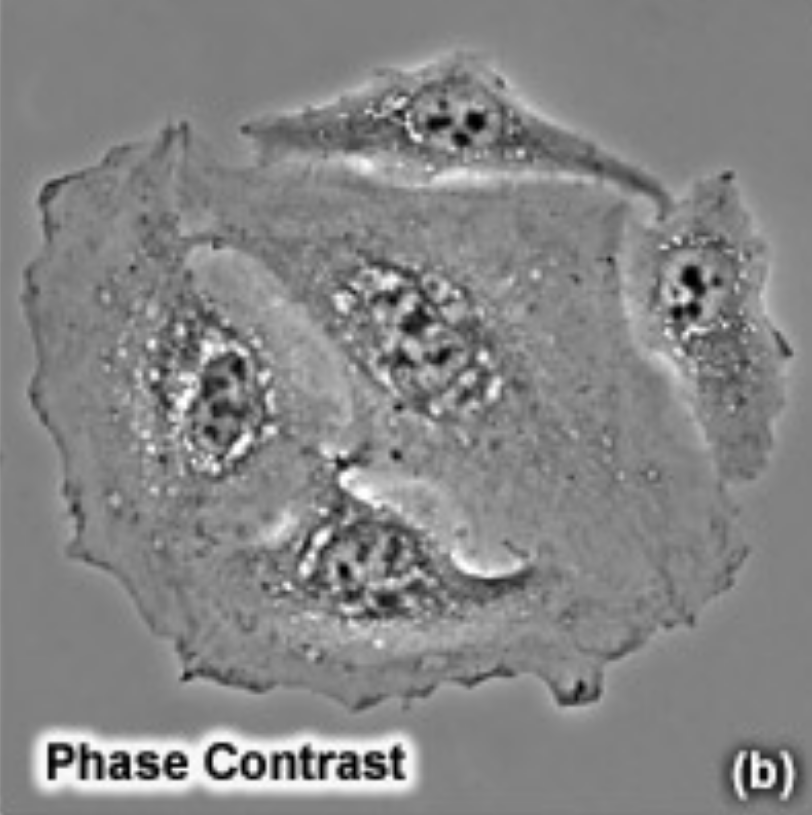
Phase-contrast microscopy
Indirect light microscopy.
Capable of viewing living specimens by using visible light and optical lenses to magnify an image.
Two complimentary out-of-phase light filters can be affixed to the microscope to provide significant specimen contrast, eliminating the need for staining or fixation. The first filter only allows perimeter light to pass through, whilst the second filter blocks perimeter light. As a result, only indirectly refracted perimeter light can illuminate the specimen, resulting in enhanced edge contrast.
Haloes for phase contrast due to edge lighting
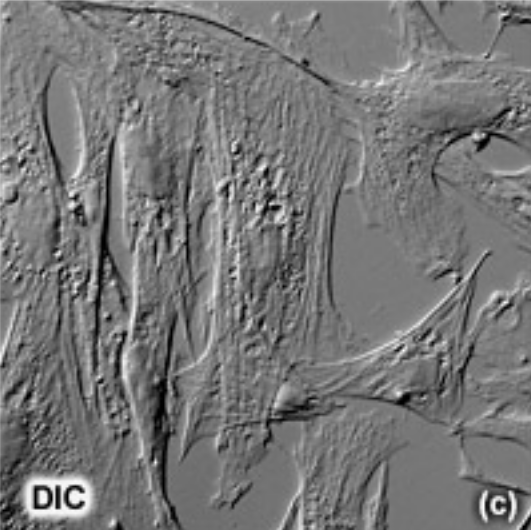
Nomarski imaging (Differential Interference Contrast)
Modified direct light microscopy.
Capable of viewing living specimens by using the phase changes of visible light and optical lenses to magnify an image.
A special objective lenses can be affixed to the microscope to observe the phase changes that light undergoes when it passes through a material with differential refractive indexes, allowing us to observe the cell in 3 dimensions with the Z axis defined by the refractive index differences.
No haloes with DIC
Fluorescence microscopy
Standard direct light microscopy, but can be combined with most techniques.
Capable of viewing internal mechanisms of living specimens using fluorescent dyes that emit light upon excitation.
Samples are labelled with fluorescent molecules that bind to specific cellular components (via antibodies), which absorb light at one wavelength and emit it at another upon excitation.
Two complimentary optical filters can be affixed to the microscope to exclusively view the emitted fluorescence. The first filter only allows excitatory light to pass through, whilst the second filter only allows fluorescent light to pass through. As a result, only fluorescently-tagged structures in the specimen are observed.
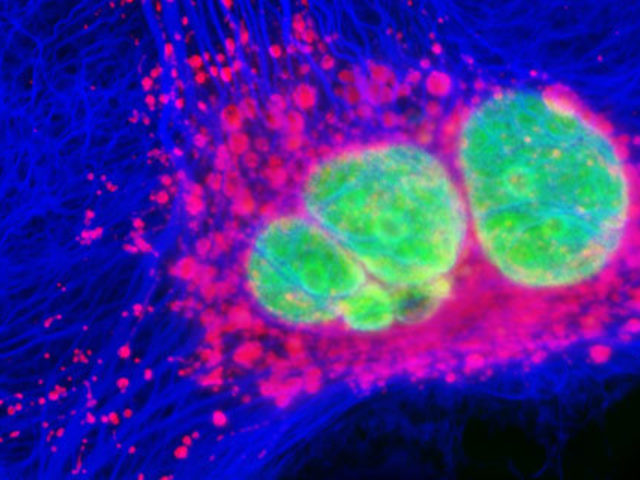
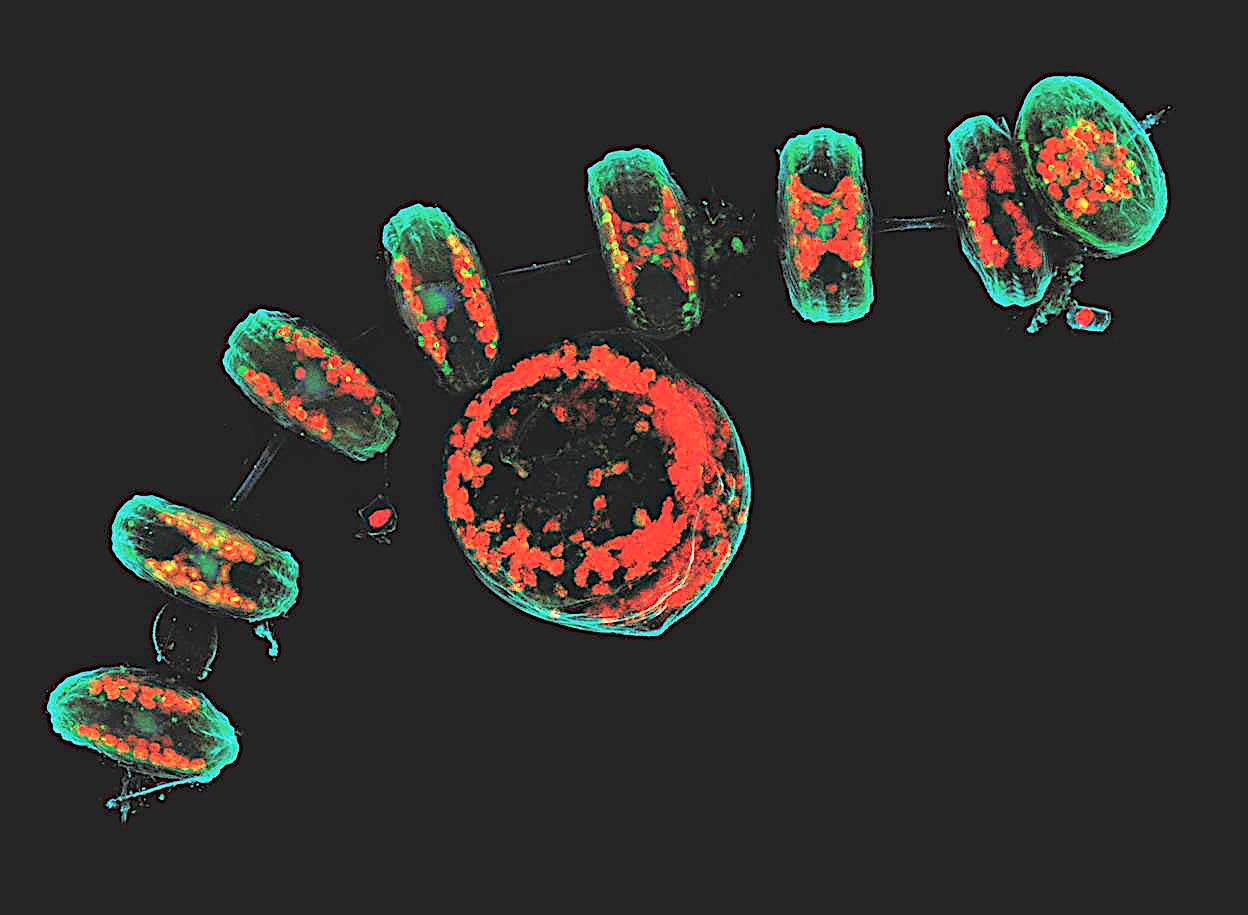
Confocal microscopy
Optical sectioning and reconstruction microscopy.
Capable of pinpointing fluorescence-tagged internal mechanisms of living specimens using a focused laser beam to scan the specimen point by point, creating high-resolution 3D images.
This technique enhances contrast and provides three-dimensional images by capturing multiple optical sections at different depths using a pinhole aperture to eliminate out-of-focus light, combined with a computer to reconstruct the images recorded by the laser scanner.
Can trace things but at a limited speed