chromatography
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
what can high resolution mass spectrometry be used for?
High resolution mass spectrometry can be used to deduce / confirm molecular formulae
High resolution mass spectrometers provide data accurate to four, or sometimes more, decimal places
This means that a compounds molecular formula can sometimes be deduced by using atomic masses accurate to four decimal places
what are the three analytical technique used to separate the components of a mixture
simple (inc thin layer)
liquid and HPLC
Gas
how simple chromatography seperates compounds?
chromotography works because a mobile phase sweeps over the stationary phase
the components seperate as the solvent moves over the paper, due to different affinities to the mobile and stationary phase
a greater affinity for the stationary phase means the component moves up the paper more slowly
how to calculate retardation factor?
distance moved by component/ distance moved by solvent
results compared to book values
problems with chromotography
similar substances have similar Rf values
new (novel) structures have nothing to be compared with
It can be difficult to manage the conditions, e.g. temperature and pressure
use of thin layer chromatography
Thin-layer chromatography (TLC) is a technique used to analyse small samples via separation
For example, we could separate a dye out to determine the mixture of dyes in a forensic sample
how does thin layer chromatography
a thin layer of an adsorbent material such as silica gel or alumina are supported on a glass/plastic sheet
The solute molecules adsorb onto the surface
Depending on the strength of interactions with the stationary phase, the separated components will travel particular distances through the plate
The more they interact with the stationary phase, the more they will 'stick' to it
what is stationary phase in thin layer chromatography?
solid silica on a plastic or glass plate
what is mobile phase on TLC
liquid solvent eg water or organic
Flows over the stationary phase
It is a polar or non-polar liquid (solvent) that carries components of the compound being investigated
Polar solvents - water or alcohol
Non-polar solvents - alkanes
how to locate spots on TLC if sample components are not colored
To locate the spots we can use:
UV light
Ninhydrin (carcinogenic)
Iodine vapour
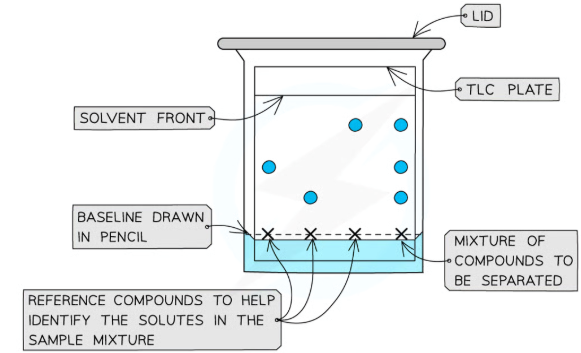
conducting a TLC analysis
Step 1:
Prepare a beaker with a small quantity of solvent
Step 2:
On a TLC plate, draw a horizontal line at the bottom edge (in pencil)
This is called the baseline
Step 3:
Place a spot of pure reference compound on the left of this line, then a spot of the sample to be analysed to the right of the baseline and allow to air dry
The reference compounds will allow identification of the mixture of compounds in the sample
Step 4:
Place the TLC plate inside the beaker with solvent - making sure that the solvent does not cover the spot - and place a lid to cover the beaker
The solvent will begin to travel up the plate, dissolving the compounds as it does
Step 5:
As solvent reaches the top, remove the plate and draw another pencil line where the solvent has reached, indicating the solvent front
The sample’s components will have separated and travelled up towards this solvent front
how to carry out column chromatography?
The sample mixture is dissolved in the solvent and introduced at the top of the column
A pipette is usually used to carefully add the dissolved sample to the top of the column
add the sample without disturbing the surface of the column so that the sample runs from one level through the column
Once the sample has been added, more solvent (eluent) is added on top of the sample
As the solvent runs through, fresh solvent is added to the top of the column so that it does not dry out
The sample flows through the column via gravity
how column chromatography works?
the four components leave the column separately. The component with the greatest attraction / affinity to the stationary phase takes the longest time to flow through the column. Their retention times are measured
once through each sample is analysed using a mass spectrometer
how can process of column chromatography be sped up?
This process can be sped up by pushing the sample and mobile phase through the column
In school laboratories, this can be achieved by attaching a gas syringe to the top of the chromatography column
In industrial / research laboratories, this is achieved by attaching an air line to the top of the chromatography column
what is retention time
the time taken from injection to detection
what is stationary phase for column chromatography
solid alumina or silica gel, packed into the HPLC tube
what is mobile phase in column cheomtography
usually an organic solvent or water
what is a high performance liquid chromatography?
a more elaborate column chromatography
retention times are recorded for each component in the mixture
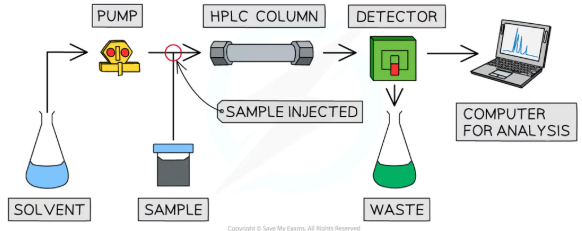
main difference between HPLC and column chromatography
The column doesn't work via gravity, the sample is pumped through by the solvent
The particles of the stationary phase are much smaller, leading to greater separation of compounds
There is a detector at the end of the column which measures retention time
HPLC is automated so the results are obtained quicker
The HPLC equipment typically includes a computer which allows for quicker analysis and comparison of results against known compounds in a database
how gas chromatography works?
after injection,into the column through a self-sealing disc the sample vaporises and the vaapour formed in arried throught eh statinary phase using the inert-gas mobile phase
the mobile carrier gas carries the mixture through the column
Once sample molecules reach the detector, their retention times are recorded
The retention times are recorded on a chromatogram where each peak represents a volatile compound in the analysed sample
The relative sizes (i.e. areas) of the peaks are related to how much of each compound is present in the mixture
components more strongly attracted to the stationary phase have longer retention times
Retention times are then compared with data book values to identify unknown molecules
what is G.c- M.S
Mass spectrometry is combined with gas chromatography to roduce a more powerful analytical tool
G.C- seperates the components
M.S- identifies each component ( molecular ion for Mr and fragments for detailed structure)
Concentrations as low as 1×10^-9 mldm^-3 can be detected
uses of gas chromotography and M.S and HPLC
Provide forensic evidence
Drug testing, particularly in sports
Analysis of environmental pollution
Detecting explosives in baggage
Problems with using GC-MS for drug detection
Anabolic steroids can be used by athletes to improve muscle growth, increase production of red blood cells and strengthen bones by increasing their density
They are also used to treat conditions such as osteoporosis, anaemia and some cancers
One high profile anabolic steroid is nandrolone which is metabolised into a similar chemical called 19-norandrosterone
Competitors in the Olympic Games are routinely urine tested for the presence of 19-norandrosterone
A urine content above 2 nanograms per cm3 (0.000000002 g per cm3) is a positive test and can result in the athlete being disqualified and risking further sanctions
There is debate about nandrolone due to its genuine medical applications and the fact that it may be in some nutritional and dietary supplements
stationary phase in gas chromatography?
microscopic liquid film on a solid support
mobile phase in gas chromatography?
inert carrier gas eg nitrogen, Ar, He
what does NMR require?
low energy radio frequency radiation (radio waves)
a strong magnetic field (electromagnet)
what happens to nucleons (protons and neutrons) in the nucleus?
Nucleons pair up and spin in opposite directions, in the nucleus
protons spin interact with the spin sttes of nearby protons that are in diffrerent environments. his can provide information about the number of protons bonded to adjacent carbon atoms. The splitting of a main peak into sub-peaks is called spin-spin splitting
even number of nucleons- their spins cancel each other out and so cannot be detected
odd number of nucleons- the unpaired nucleon produces a small residual spin, that generates a magnetic field
atoms eg isotopes of atoms with odd mass numbers usually show signals on NMR
how NMR works?
when placed in a magnetic field, the nucleon can flip and spins in the opposite direction when they absorb energy from the radiowaves
the energy absorbed is measured and used to plot a graph
Carbon-12 cannot be detected with 6 P and 6N, it has an even number of nucleons
Carbon-13, 6P and 7N or hydrogen-1 (1P and 0N) are mainly used, but 15N, 19F, and 31P are also used
what is the solvent in NMR?
the sample is dissolved in a solvent that must not produce a signal
what is the references for NMR
all NMR spectra are compared to a reference molecule- tetramethysilane, TMS
TMS shows a single sharp peak on NMR spectra, at a value of zero
TMS is also used because it is:
Non toxic.
Does not react with the sample.
Easily separated from the sample molecule due to its low boiling point.
Produces one strong, sharp absorption peak on the spectrum.
what is chemical shift
describes the displacement of the signal for the sample, to the reference peak
Identical atoms (carbon or Hydrogen) in the same environment absorb the same frequency of radiation
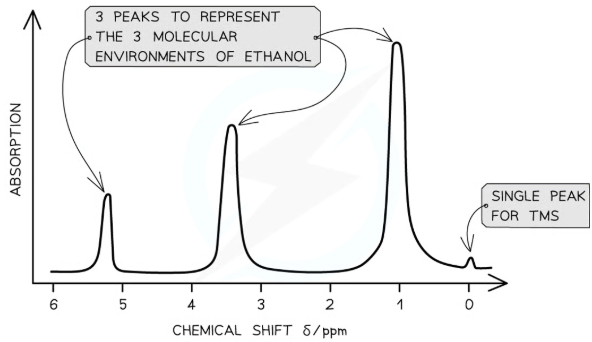
features of H1 NMR spectrum
NMR spectra shows the intensity of each peak against their chemical shift
The area under each peak gives information about the number of protons in a particular environment
The height of each peak shows the intensity / absorption from protons
A single sharp peak is seen to the far right of the spectrum
This is the reference peak from TMS
Usually at chemical shift 0 ppm
what are moelcular environmnts
1H nuclei that have different neighboring atoms (said to have different chemical environments) absorb at slightly different field strengths
The difference environments are said to cause a chemical shift of the absorption
Different types of protons are given their own range of chemical shifts
Each individual peak on a 1H NMR spectrum relates to protons in the same environment
Protons in the same chemical environment are chemically equivalent
relative intensities of each splitting pattern
A doublet has an intensity ratio of 1:1 – each peak is the same intensity as the other
In a triplet, the intensity ratio is 1:2:1 – the middle of the peak is twice the intensity of the 2 on either side
In a quartet, the intensity ratio is 1:3:3:1 – the middle peaks are three times the intensity of the 2 outer peaks
how splitting patterns occur
The number of sub-peaks is one greater than the number of adjacent protons causing the splitting
For a proton with n protons attached to an adjacent carbon atom, the number of sub-peaks in a splitting pattern = n+1
Splitting patterns must occur in pairs, because each protons splits the signal of the other