Organic Chemistry (IB)
Unit 1: Organic Nomenclature & Structure
Homologous Series
Organic chemistry is the study of carbon-containing compounds
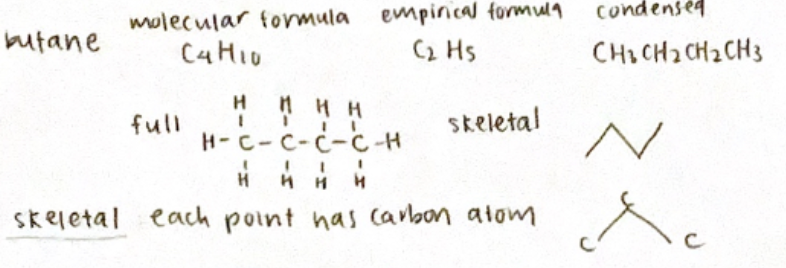
Eg: butane
molecular formula: C4H10
empirical formula: C2H5
condensed formula: CH3CH2CH2CH3
Homologous Series: Same functional group
The boiling point increases as the carbon chain goes up
Refer to the Homologous chart
For skeletal form: each point has a carbon atom
Naming Hydrocarbons
Alkanes: name (longest), then alkyl groups
Arrange names of substituent groups in alphabetical order (ignoring prefixes)
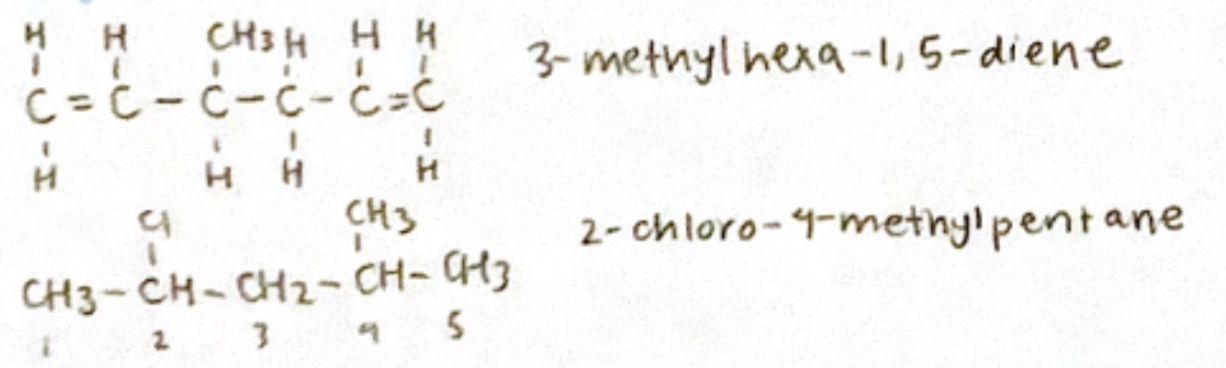
Alkenes: alk-x-ene
C=C (Double Bond)
Double bond starts at the lowest number
Alkynes: alk-x-yne
CC (Triple Bond)
Triple bond starts at lowest number
Halogen Alkane: x-halo alkane
Naming Oxygen-Containing Organic Compounds
Alcohol: Alkan-x-ol (Methanol & ethanol no need for #)
Ether: x-alkoxy alkane (treat alkoxy as substituent which is substituent with the lowest #)
give -OH the lowest #
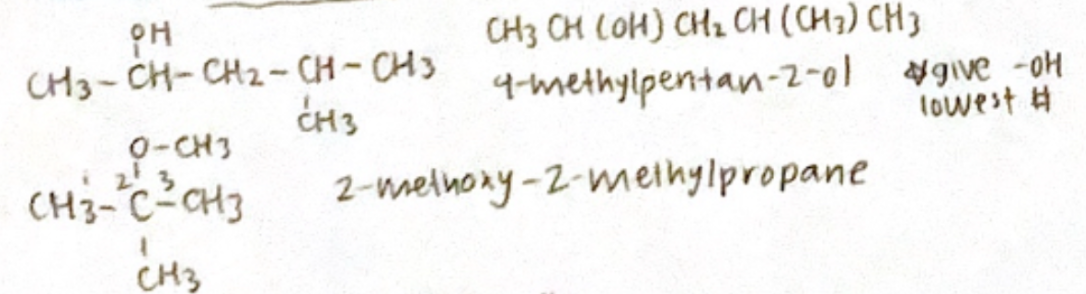
Aldehyde: alkanal, CHO is labelled #1
Ketone: Alkan-x-one, C=O group give the lowest #
no need for # for propanone or butane
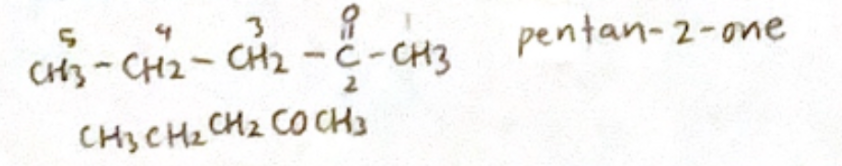
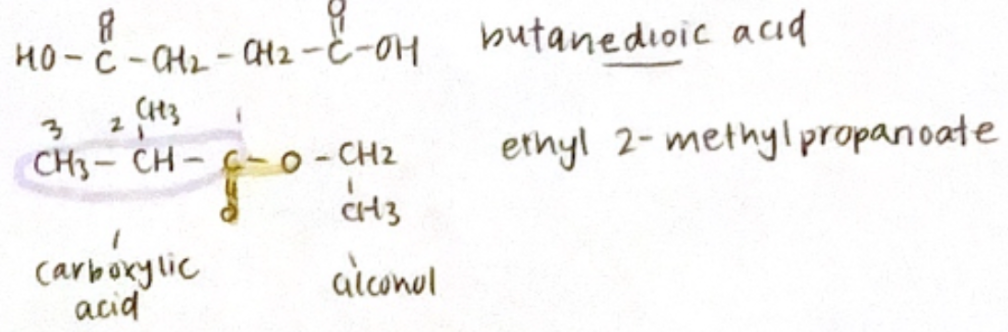
Carboxylic acid: alkanoic acid, COOH is labelled #1
Ester: carboxylic acid + alcohol
Alkyl alkanoate (alkyl for alcohol, alkanoate end for carboxylic acid side)
Numbering in the acid starts from C=O & alcohol starts from O-C group
Structual Isomers
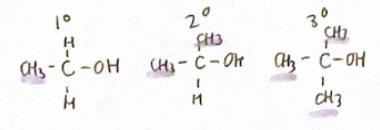
Primary, secondary & tertiary compounds
Depends on what the C attached to OH is attached to
Structural Isomers: same molecular formula but different structural formula (atoms joined together differently)
Branched-chain isomers (less surface area) have lower boiling points than straight chain isomers (high surface area, high london-dispersion forces)
Stereoisomers
Same structural formula but atoms are arranged differently in space

Cis means same side
Trans means opposite side
E/Z Priority rules (higher priority to atom attached to C=C with higher atomic #)
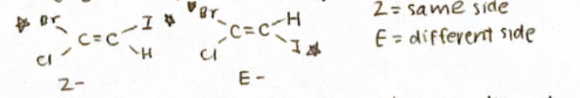
Optical Isomers: When 4 different atoms/groups attached to a single carbon atom (Chiral)
Mirrors (enantiomers)
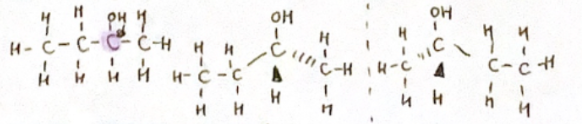
Racemic mixture: Equimolar mixture of 2 enantiomers
Unit 2: Organic Reactions & Mechanisms
Reactions of Hydrocarbons
Complete oxidation of a hydrocarbon
hydrocarbon + oxygen = carbon dioxide + water
C6H12 + 9O2 (g) = 6CO2 (g) + 6H2O (l)
When oxygen supply is limited, a hydrocarbon will undergo incomplete combustion (only form carbon if O2 extremely limited)
C3H8 (g) + 2O2 (g) = 3C(g) +4H2O(l)
Free radical substitution mechanism
Substitution of alkanes
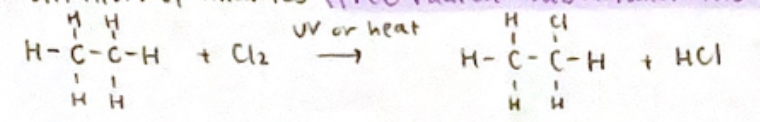
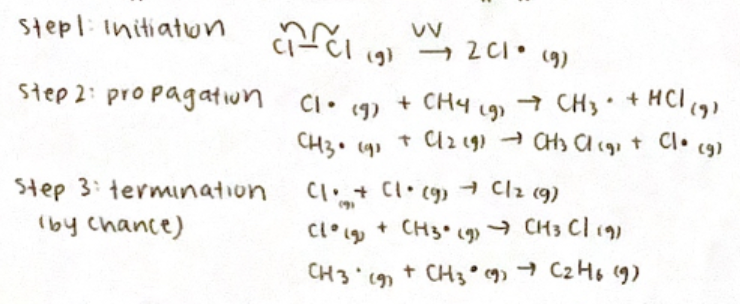
Nucleophilic substitution reaction mechanism
Nucleophilic substitution of halogenoalkanes
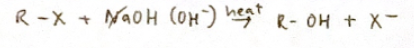
Nucleophiles: electron pair donors, attracted to electron deficient carbon atoms
Mechanisms
1° halogenoalkane (SN2 Mechanism)
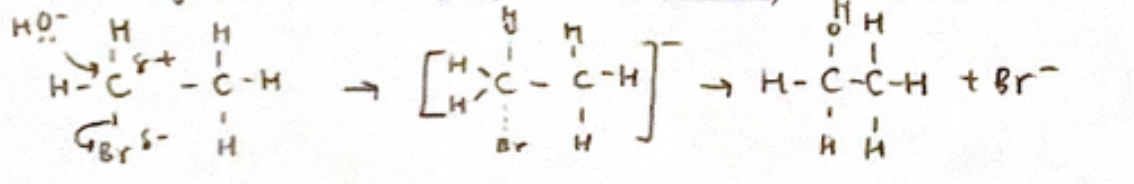 rate = k [Rx][OH-]
rate = k [Rx][OH-]inversion of configuration if a nucleophile attacks a chiral centre
3° halogenoalkane (SN1 Mechanism)
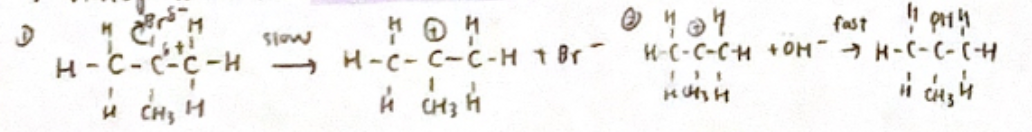 rate = k [RA]
rate = k [RA]not stereospecific - racemix mixture formed
2° halogenoalkanes undergo a mixture of Sn1 and Sn2 mechanisms
Factors that affect the rate of nucleophilic substitution reaction
Identity of nucleophile (only Sn2 reaction affected)
Anions more reactive than neutral species
Identity of halogen (Sn1 & Sn2)
1°, 2°, 3° halogenoalkane
Sn2 ratio is 1°>2°>3°
Sn1 rate is 3°>2°>1°
Choice of Solvent
Electrophilic addition mechanism
Electrophile: electron-deficient species
Attracted to regions of relatively high electron density

Halogenation
reaction with Br2
alkanes will have no reaction because it requires heat as a catalyst. therefore it will remain red
alkenes will go colourless because it does not require a catalyst
Markovnikov’s Rule
if more than one product is possible, the more electronegative atom will end up on the carbon atom of the double cond that has fewer hydrogen
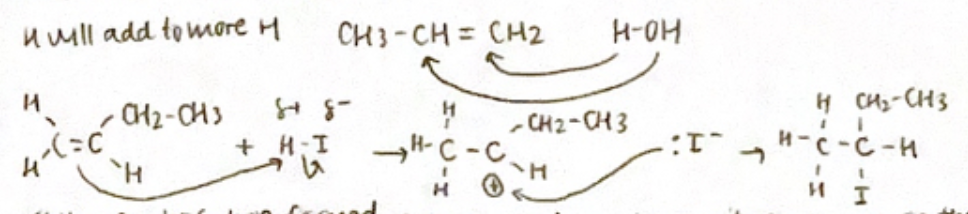
the carbocation formed is one that has its positive charge on the most substituted carbon
Electrophilic substitution mechanism
Electrophilic substitution of benzene
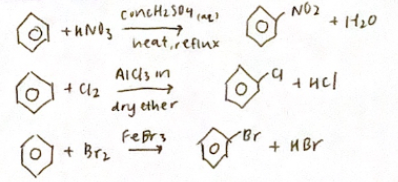
Step 1: Generation of nitronium iron (NO2+)
Mixing nitric acid with sulphuric acid at SO°C generates a higher (NO2+) mechanism
Step 2: Rate determining step
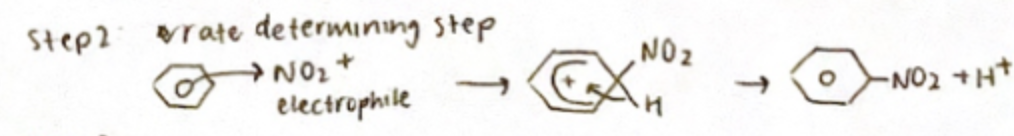
Step 3:
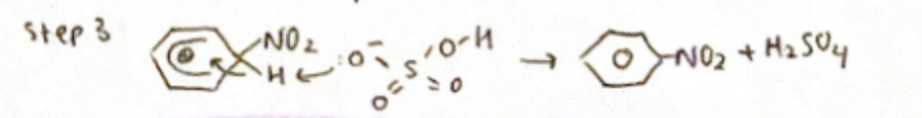
Addition polymerization of alkenes
Reactions of Oxygen-containing Compounds
Mild oxidation
(Controlled oxidation of an alcohol to create other functional groups)
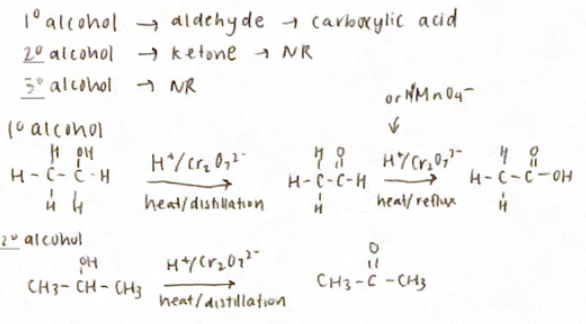
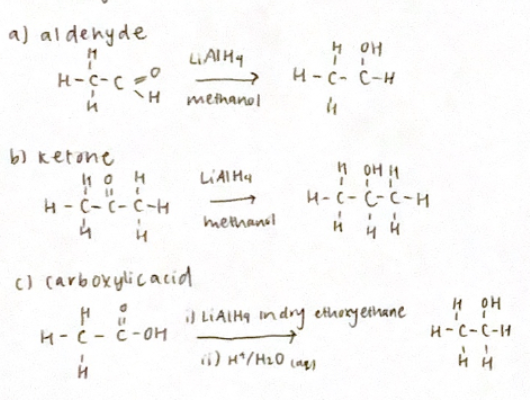
Reduction reactions
(reverse oxidation reactions)
Reducing agents include lithium aluminum hydride (LiAlH4 (This is stronger) and NaBH4
Reduction of Nitrobenzene
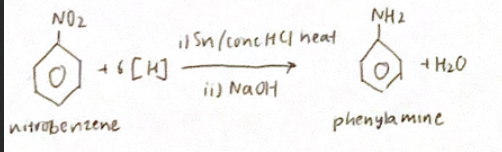
Formation of ester (condensation reaction)
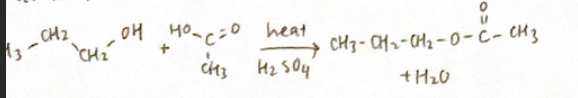
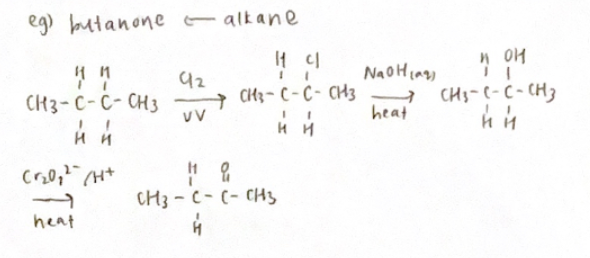
Organic Synthesis
Unit 3: Spectroscopy
IR Spectroscopy
Index of hydrogen deficiency (IHO)
Double bond = 1
Triple bond = 2
Ring = 1
Aromatic ring = 4
IHO = ½ (2C + 2 - h - x + n)
IR is absorbed by certain bonds causing them to stretch or bend
Bond will only interact with IR radiation if it is polar
Match wavenumbers with bonds
Fingerprint region (1500-650 cm^-1) difficult to interpret, lots of C-C & C-H bond vibrations
Mass Spectrometry
Measures relative masses of atoms or ions
Measures mass-to-change ratio of ions
Ionization causes molecule to break up into different fragments
Greatest mass peak is parent ion (Molecular mass)
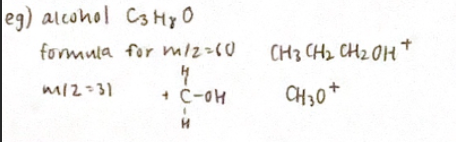
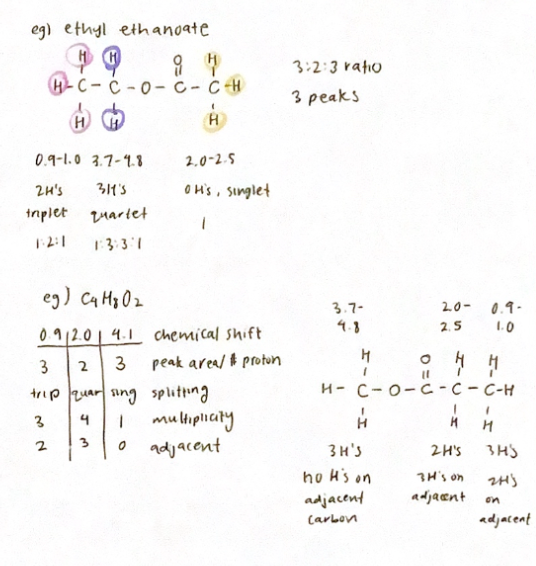
‘H NMR Spectrometry
Nuclei in different chemical environments produce different signals in the spectrum
Signals are measured against the standard signal produced by TMS (8=0 ppm)
Why TMS?
12 protons, all in the same environment
Strong signal even when present in small amounts
Chemical shift value very low
The area under a peak is proportional to the number of proton atoms in that environment
Integration trace distance/height of each step is ratio between # of protons in each environment
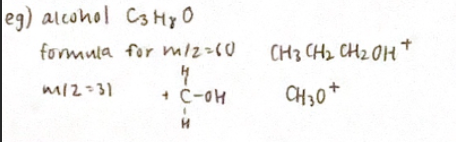
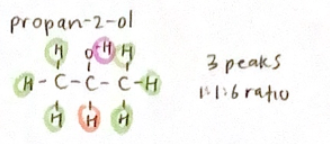
If there are n H’s on an adjacent atom, the signal for a particular proton will be split into n+1 peaks
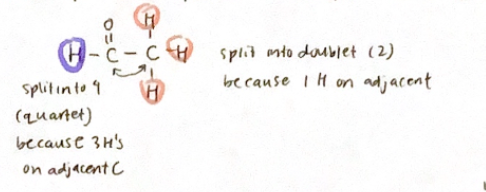
Intensities of peaks are given by Pascal’s Triangle

example