VSEPR structures, angles, polarity shaping, and intermolecular forces
1/35
Earn XP
Description and Tags
i'm gonna fart bruh
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
(ideal) 2 electron domains make...
(Ideal) Electron Geometry: Linear shape
180 degrees, nonpolar
(sp hybridization orbitals)
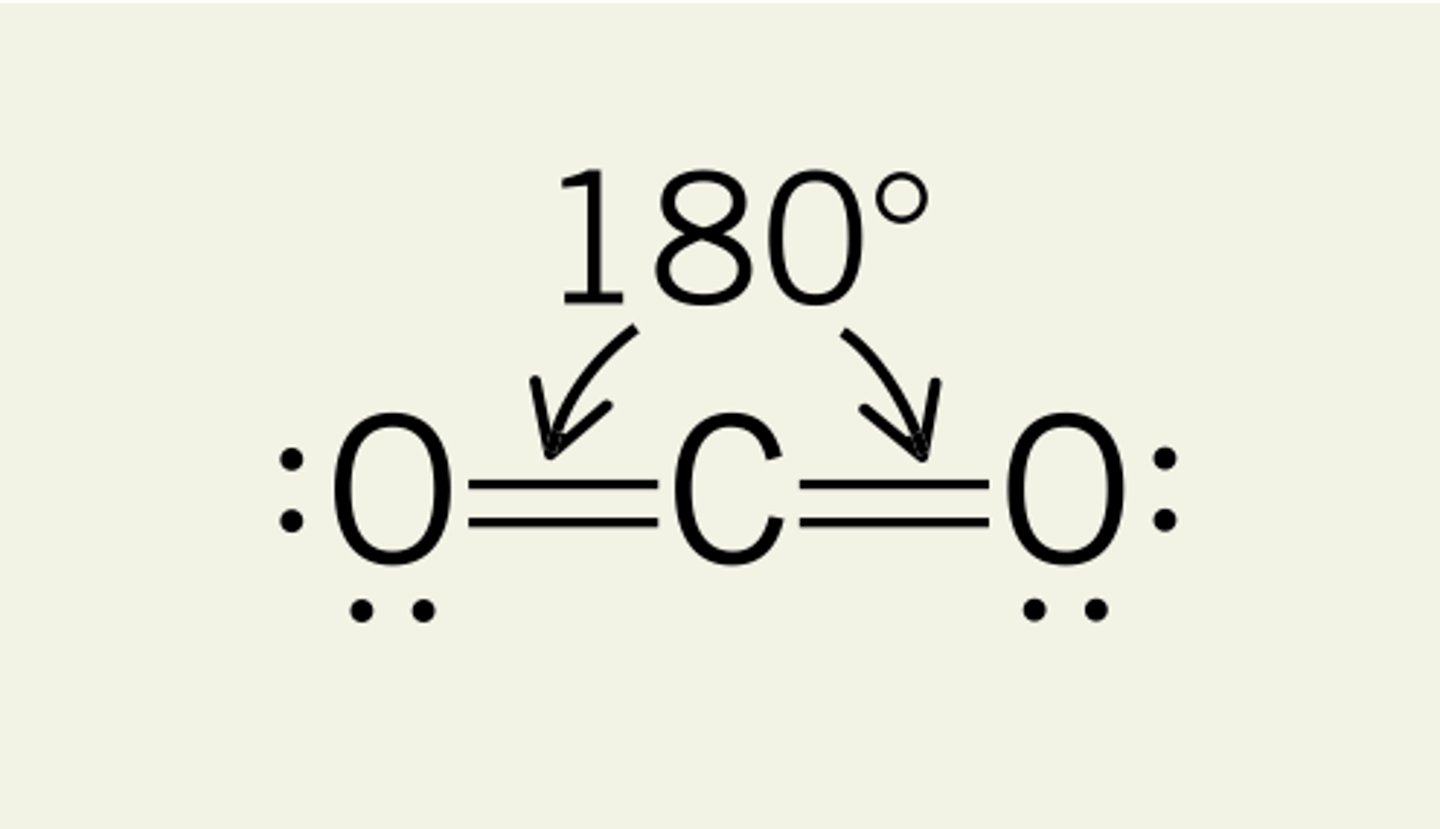
(ideal) 3 electron domains make...
(Ideal) Electron Geometry: Trigonal planar
120 degrees, nonpolar
(sp2 hybridization orbitals)
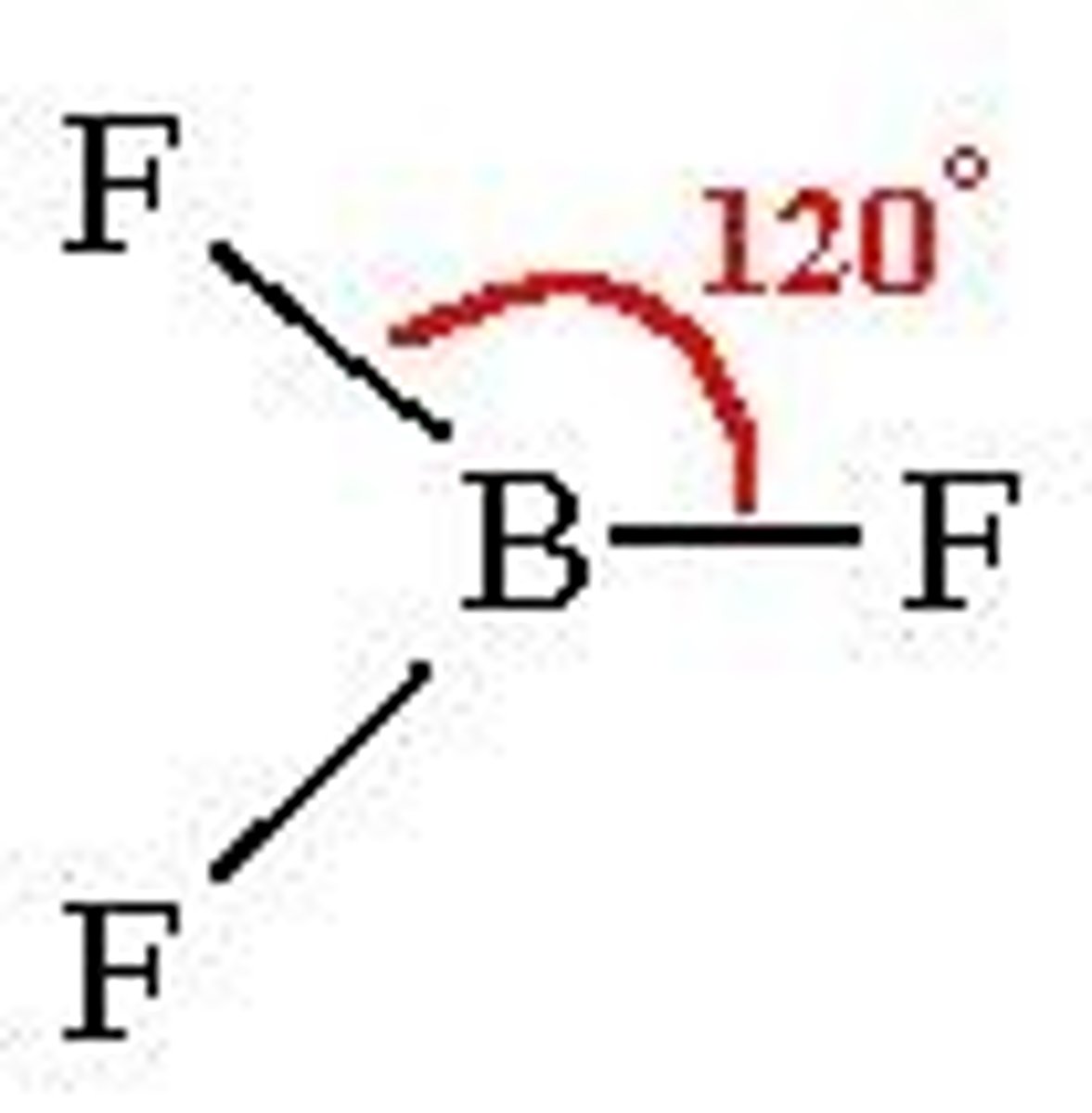
(ideal) 4 electron domains make...
(Ideal) Electron Geometry: Tetrahedral
109.5 degrees, nonpolar
(sp3 hybridization orbitals)
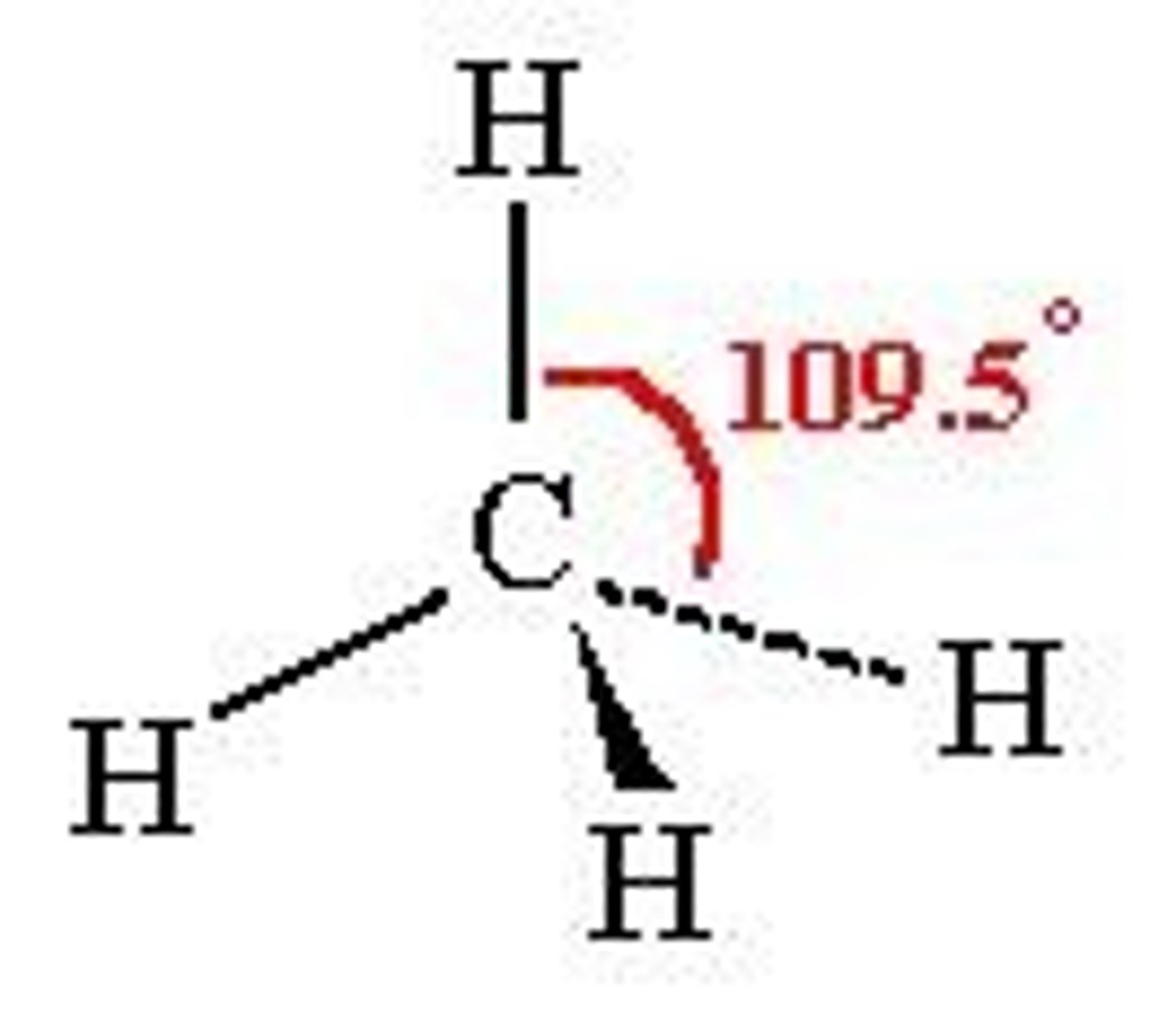
(ideal) 5 electron domains make...
(Ideal) Electron Geometry: Trigonal bipyramidal
120 degrees between the 3 equatorial atoms, 90 degrees between one equatorial atom and an axial atom
nonpolar
(sp3d hybridization orbitals)
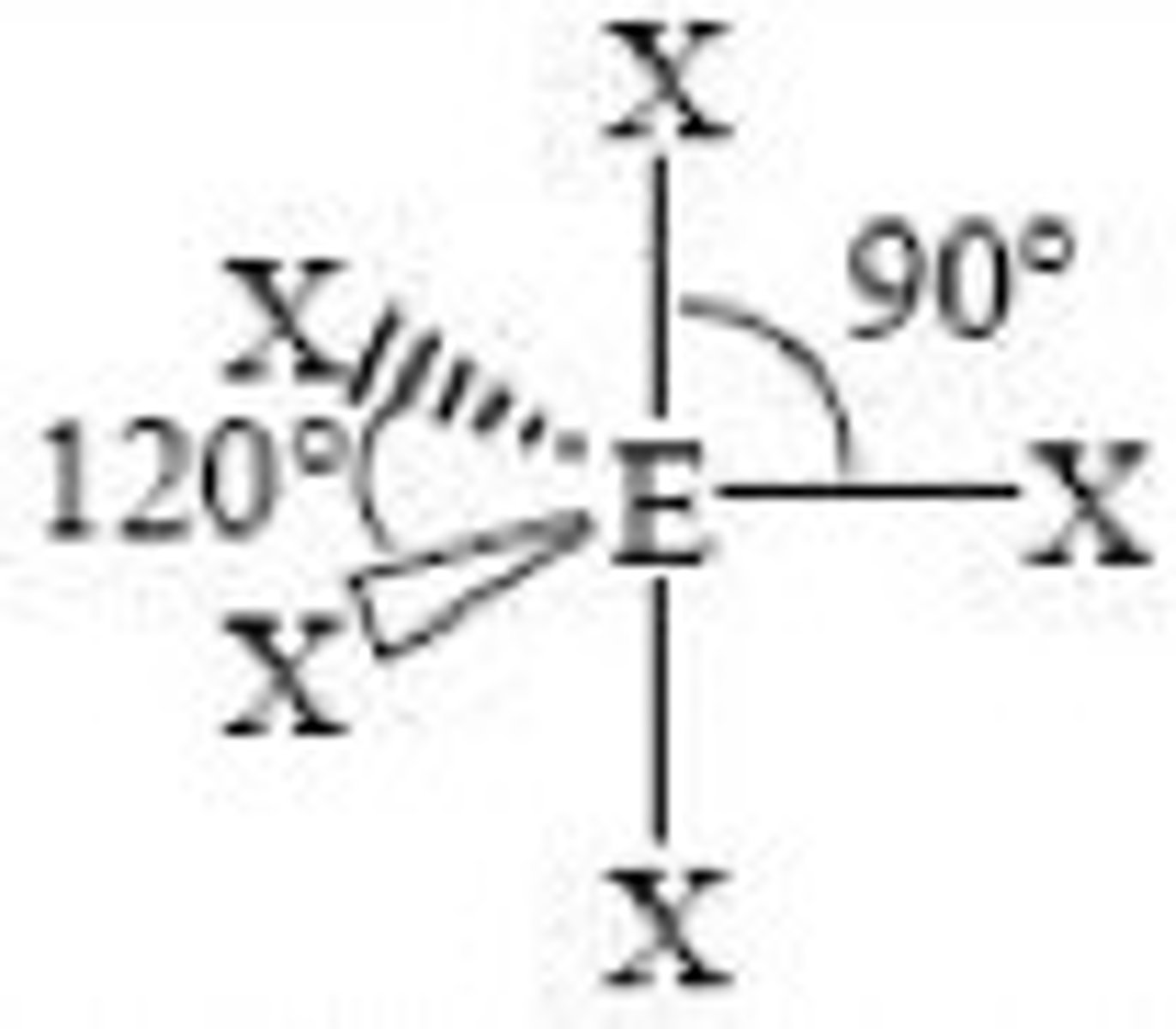
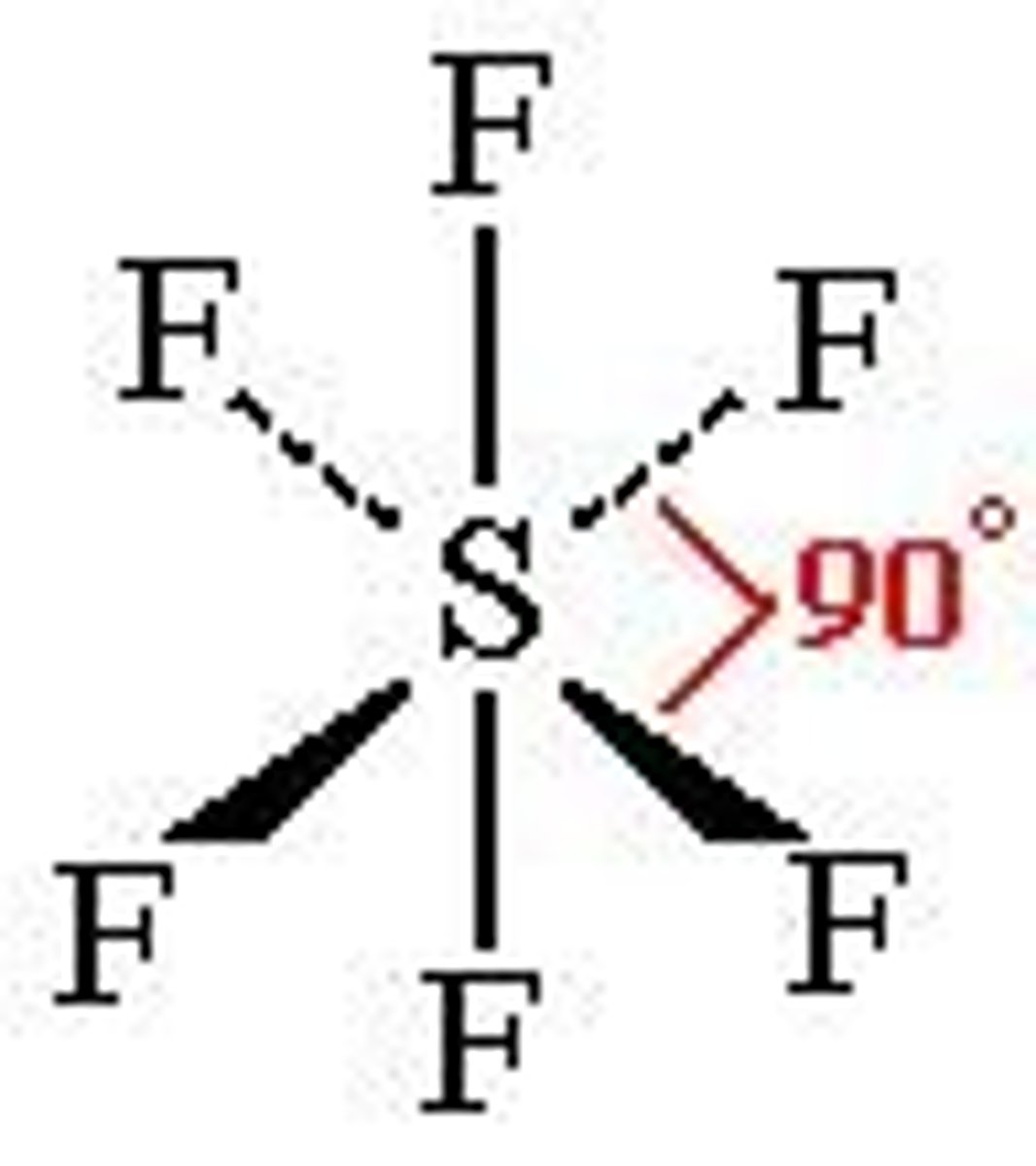
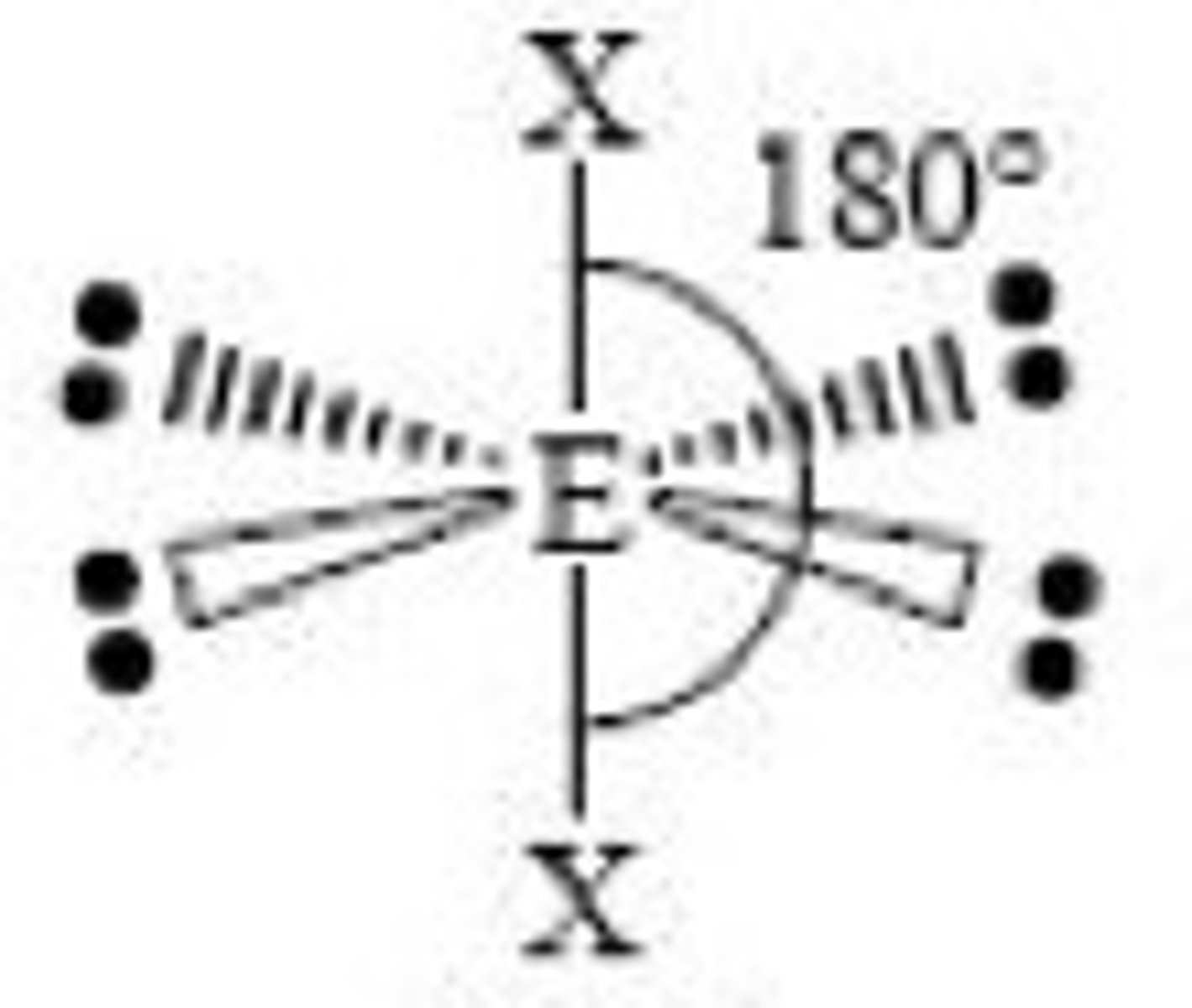
(ideal) 6 electron domains make...
(Ideal) Electron Geometry: Octahedral
90 degrees, nonpolar
(sp3d2 hybridization orbitals)
Molecular shapes are not always ideal due to...
Lone pair electrons on the central atom and to a lesser extent, double and triple bonds
Why? They take up more electronegative space and push the other terminal atoms away.
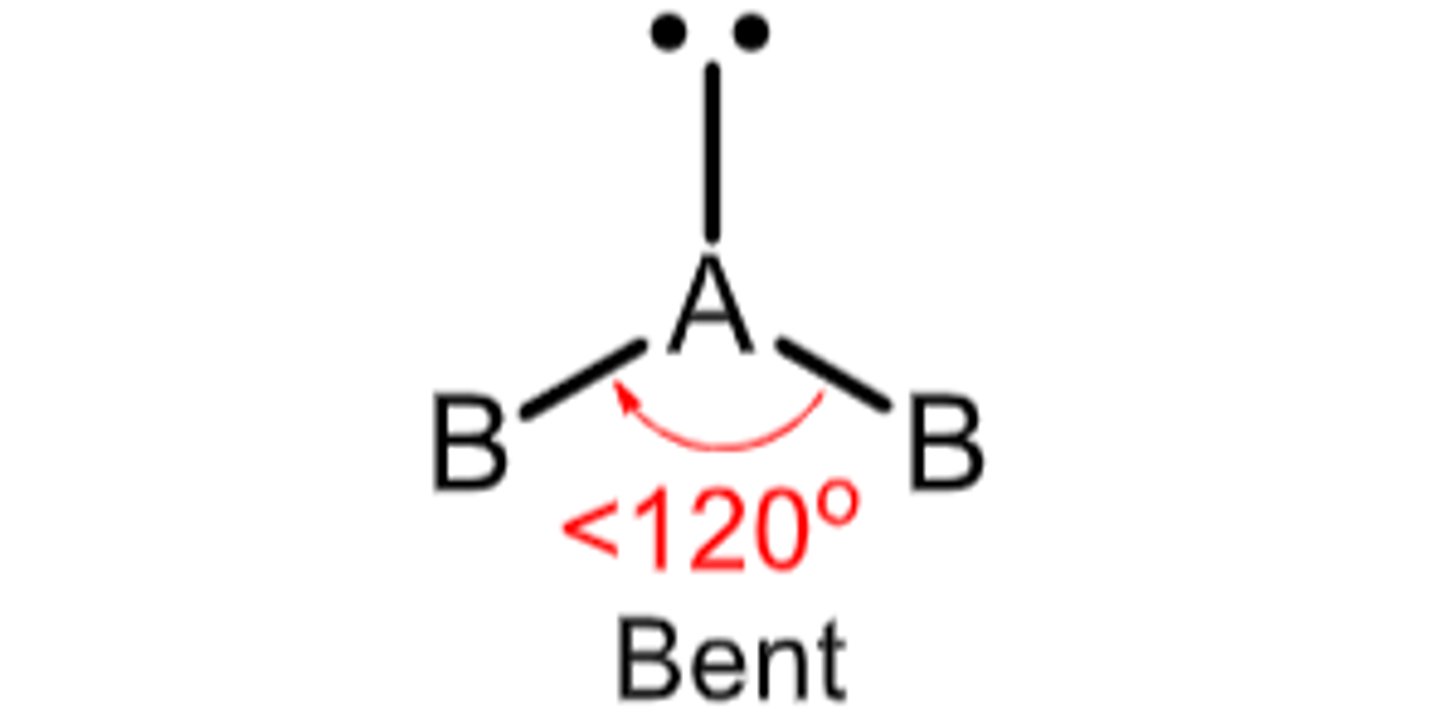
3 electron domains with 1 being a lone pair...
Molecular Geometry: Bent or Angular
less than 120 degrees, polar
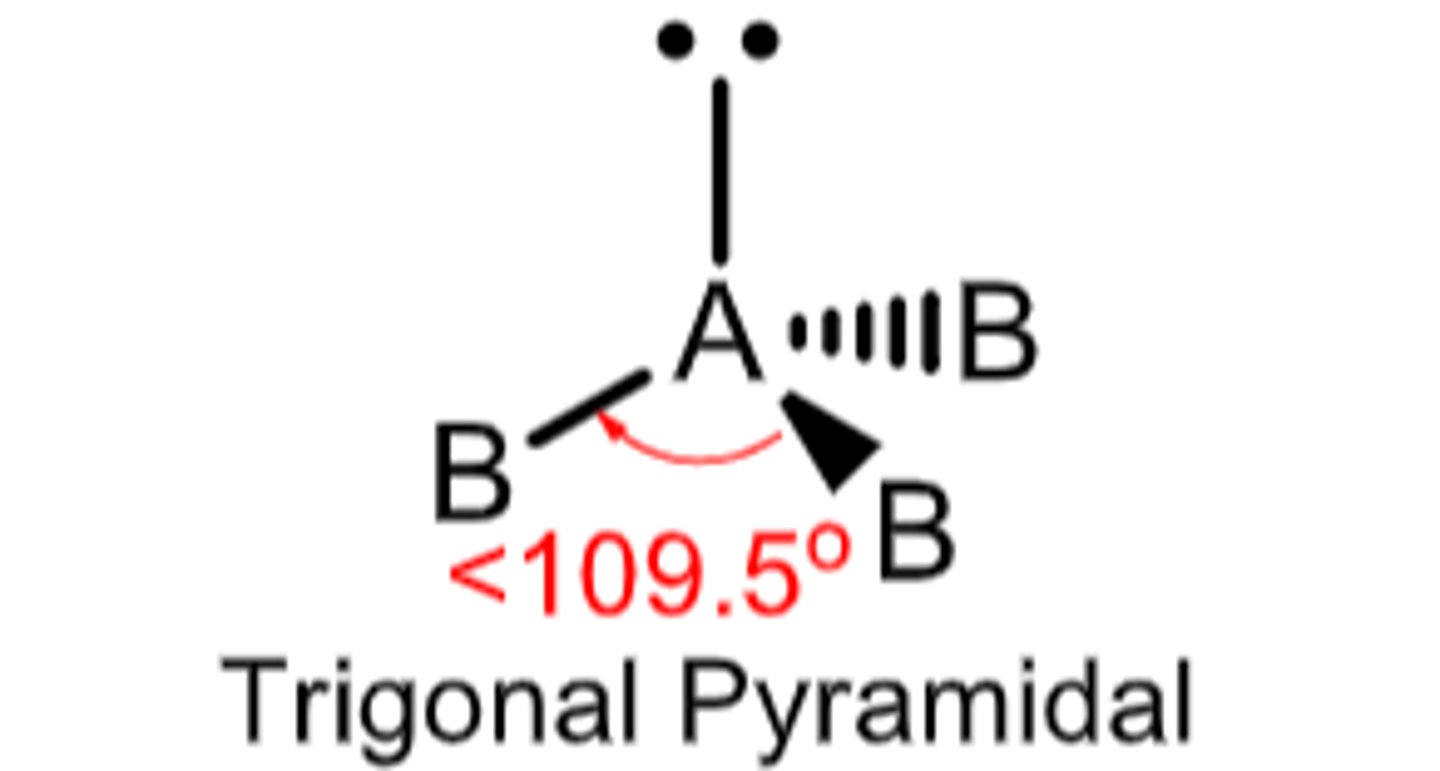
4 electron domains with 1 being a lone pair...
Molecular Geometry: Trigonal pyramid
less than 109.5 degrees, polar
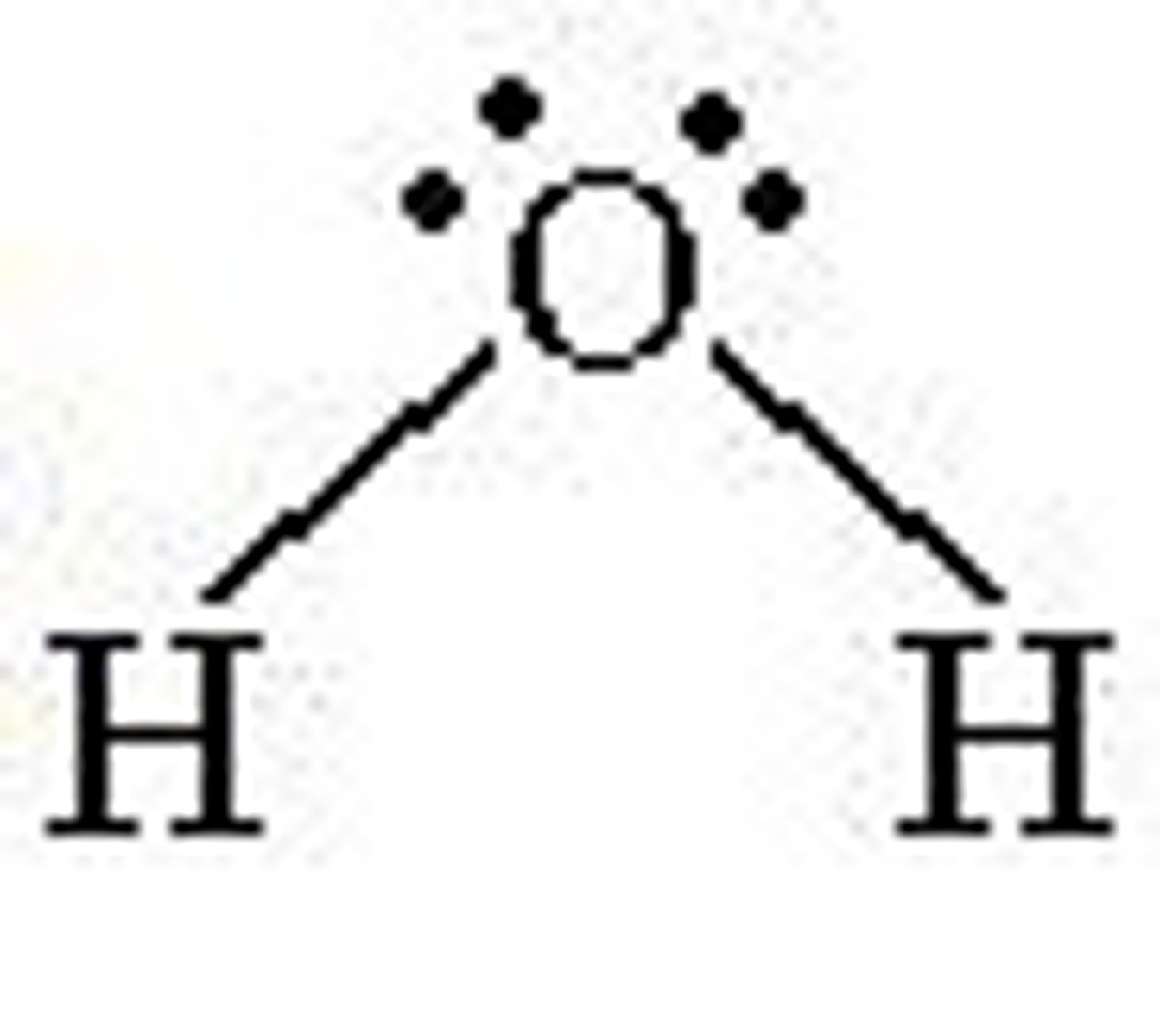
4 electron domains with 2 being lone pairs...
Molecular Geometry: Bent or Angular (H2O)
much less than 109.5 degrees, polar
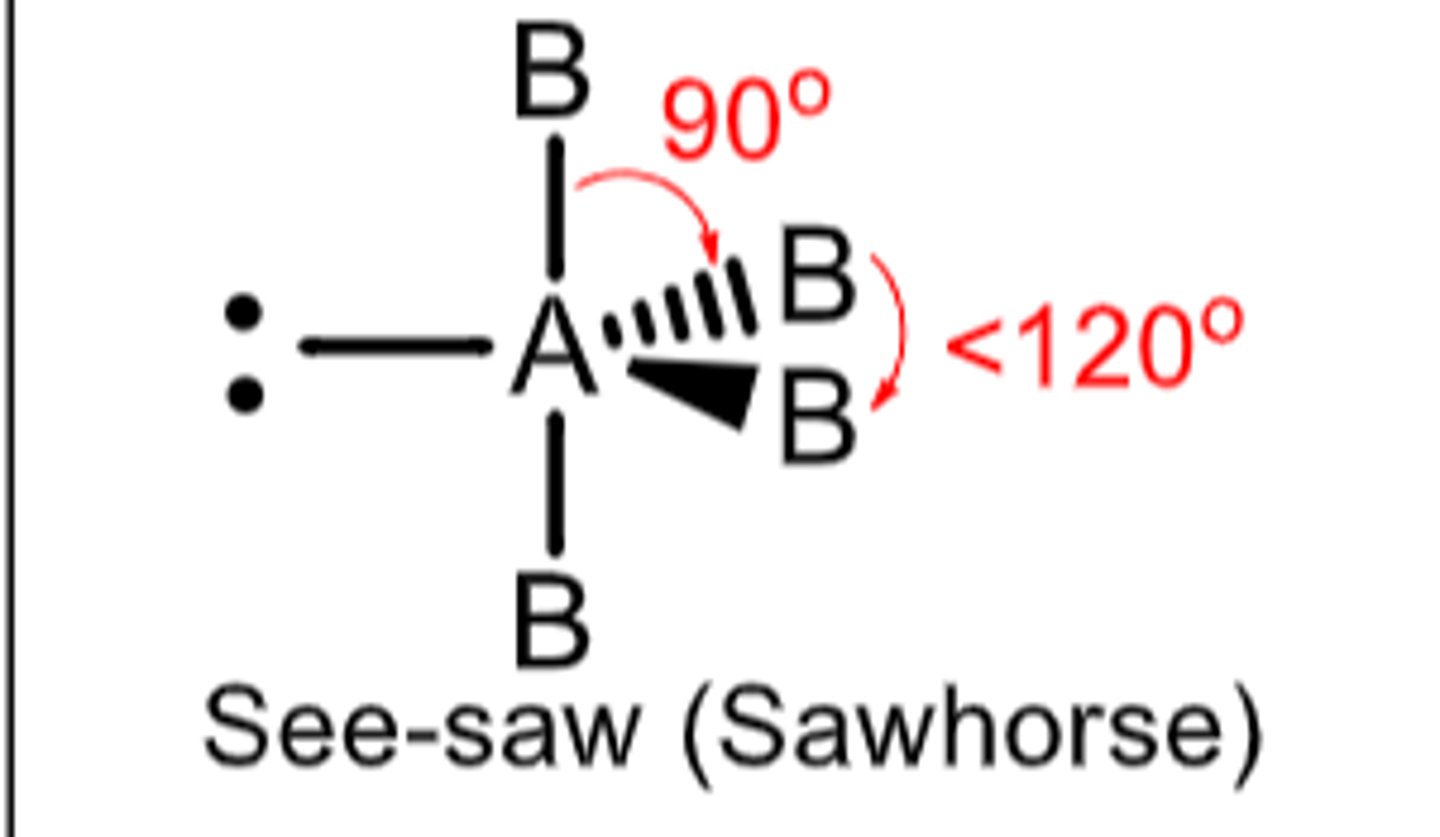
5 electron domains with 1 being a lone pair...
Molecular Geometry: Seesaw
equatorial atoms have less than 120 degrees
equatorial to axial atoms have less than 90 degrees
polar
5 electron domains with 2 being lone pairs...
Molecular Geometry: T-shape
less than 90 degrees, polar
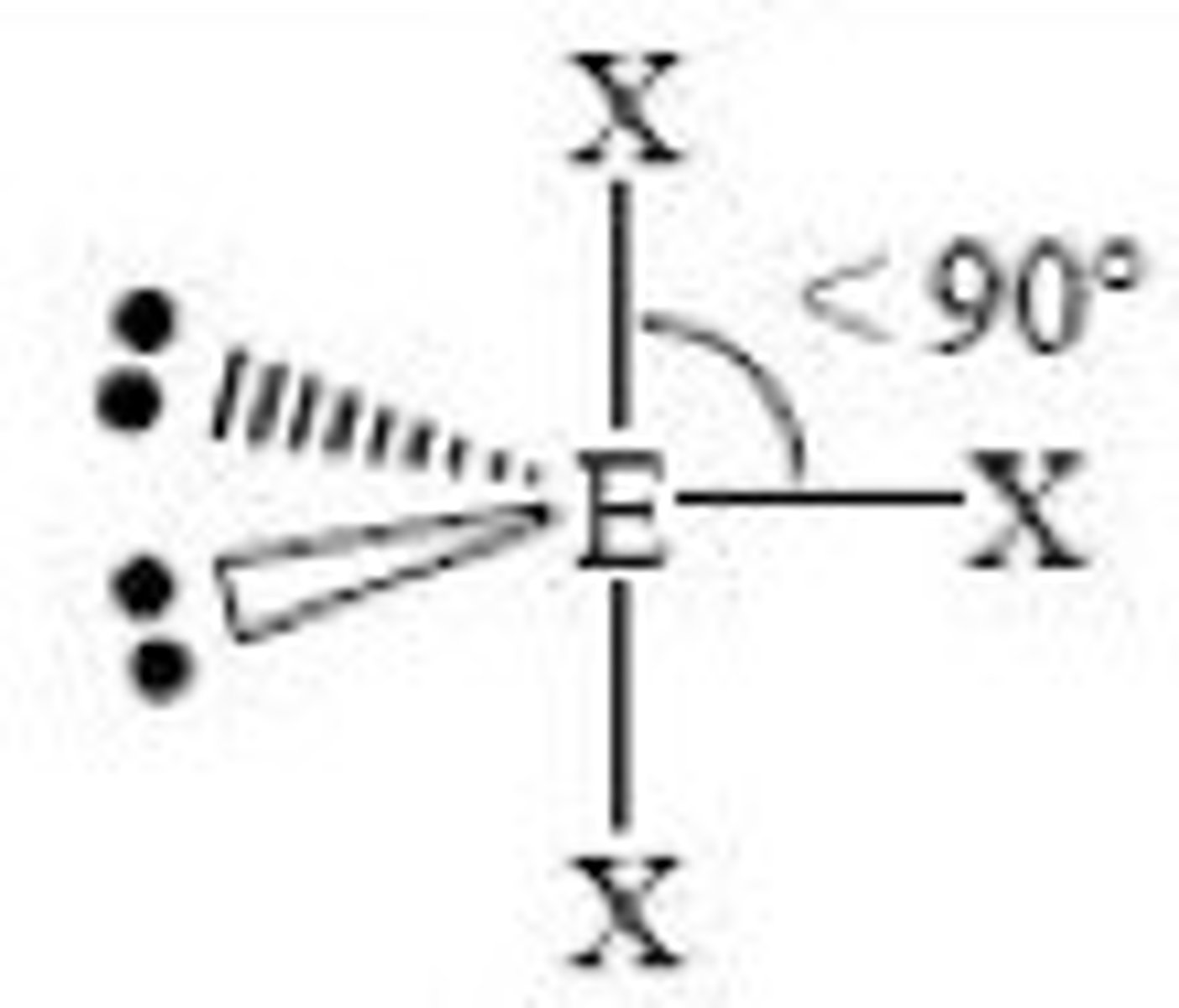
5 electron domains with 3 being lone pairs...
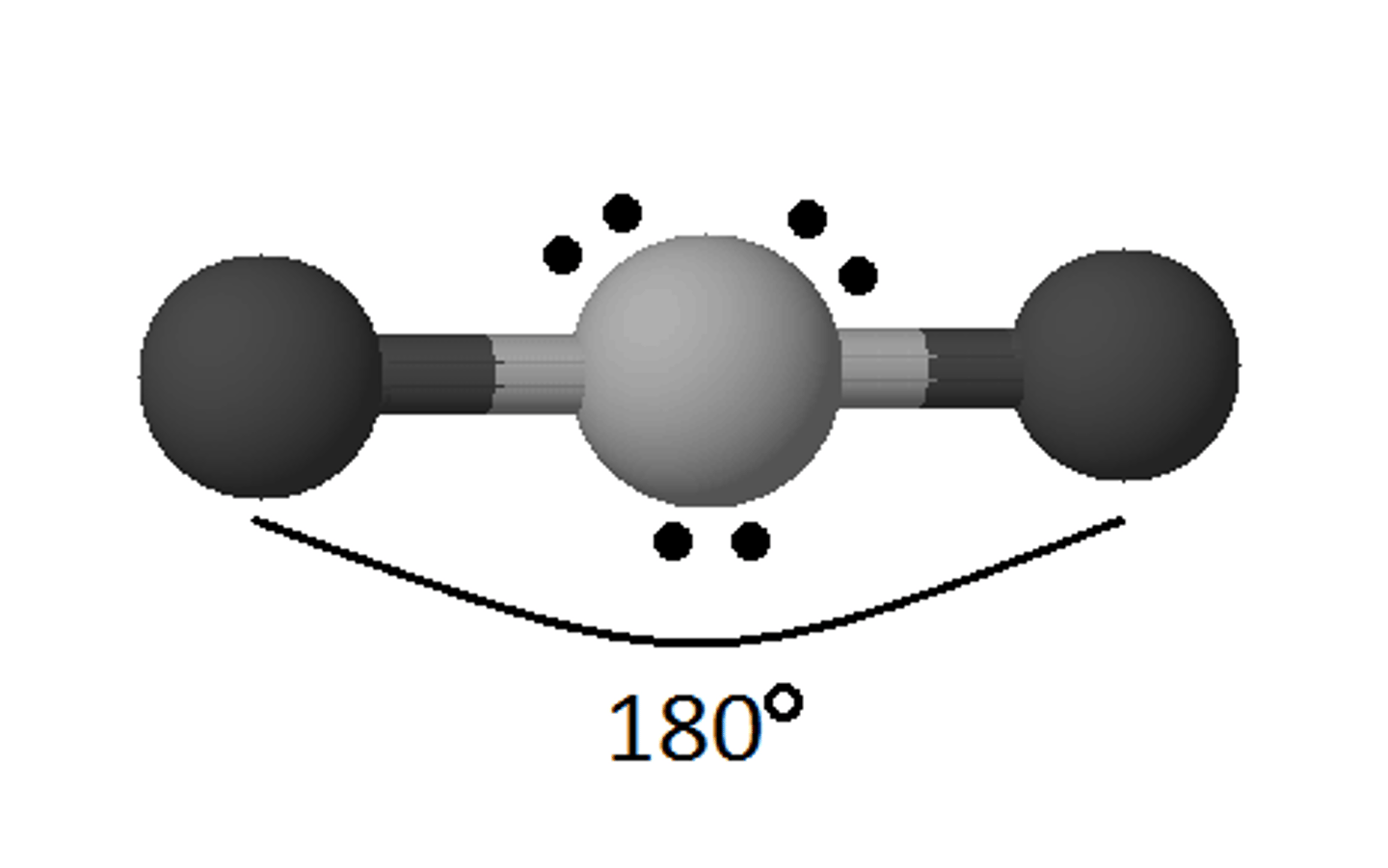
Molecular Geometry: Linear
180 degrees, nonpolar
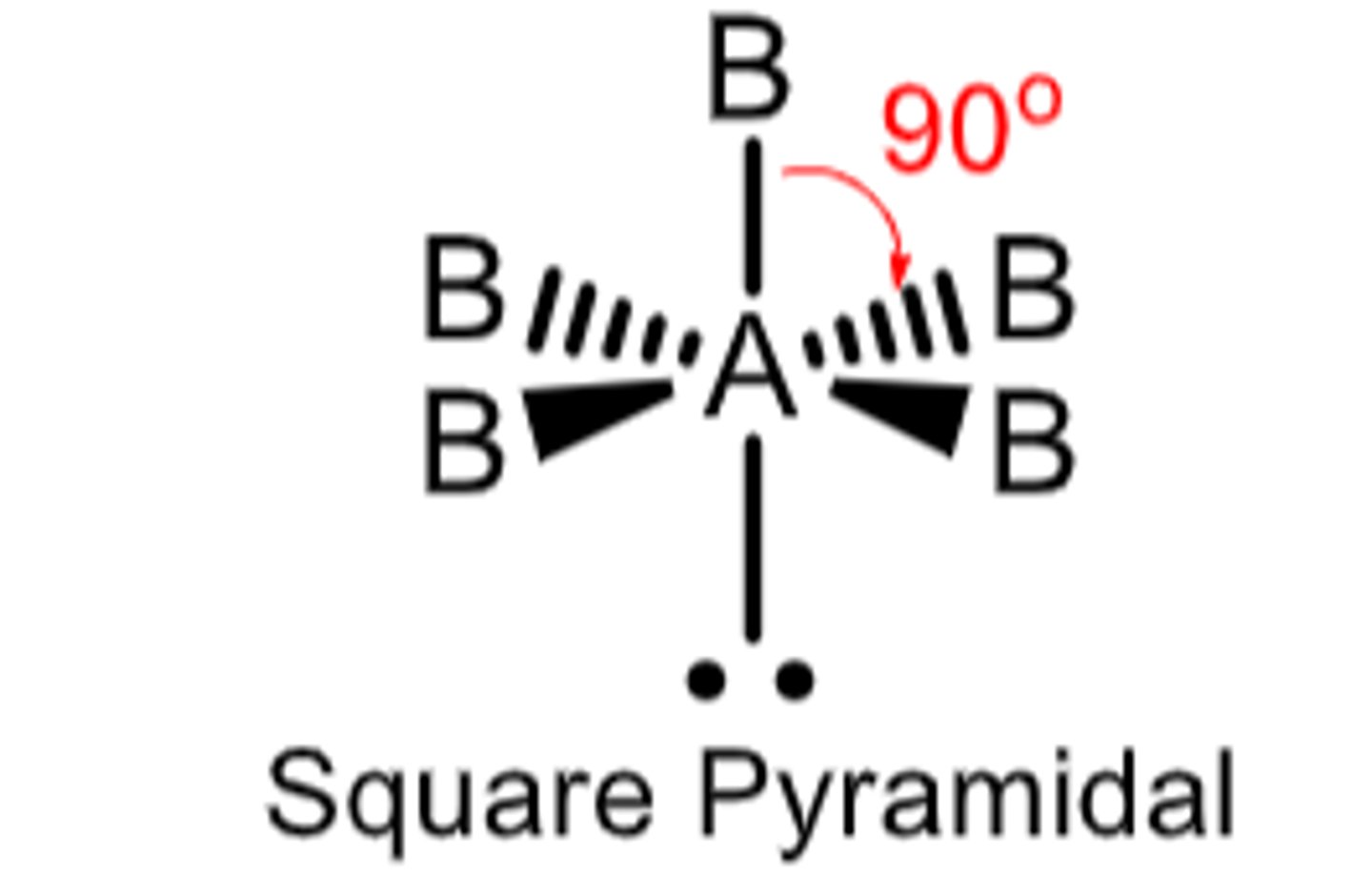
6 electron domains with 1 being a lone pair...
Molecular Geometry: Square pyramid
less than 90 degrees, polar
6 electron domains with 2 being lone pairs...
Molecular Geometry: Square planar
90 degrees, nonpolar
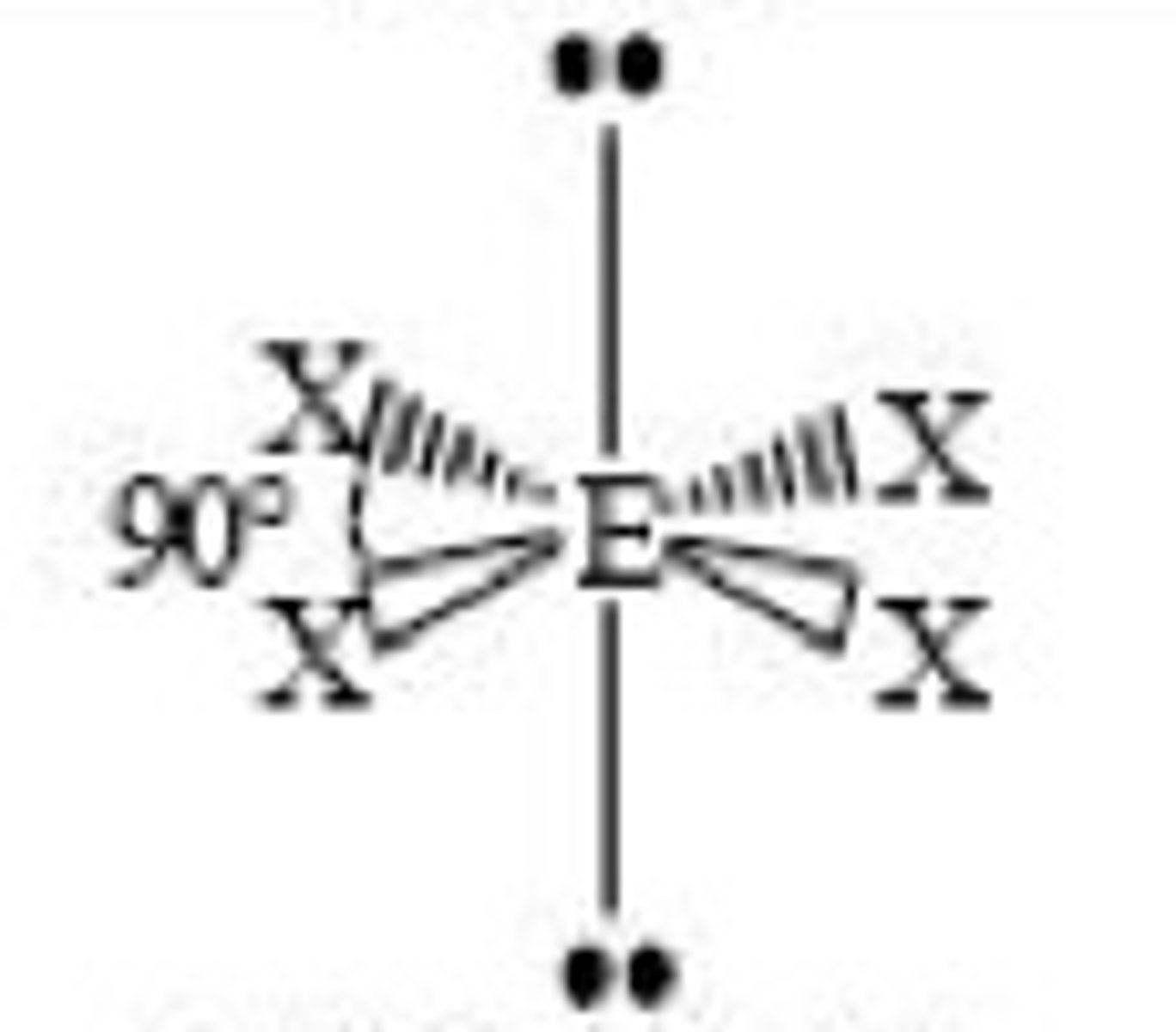
6 electron domains with 3 being lone pairs...
Molecular Geometry: T-shape
less than 90 degrees, polar
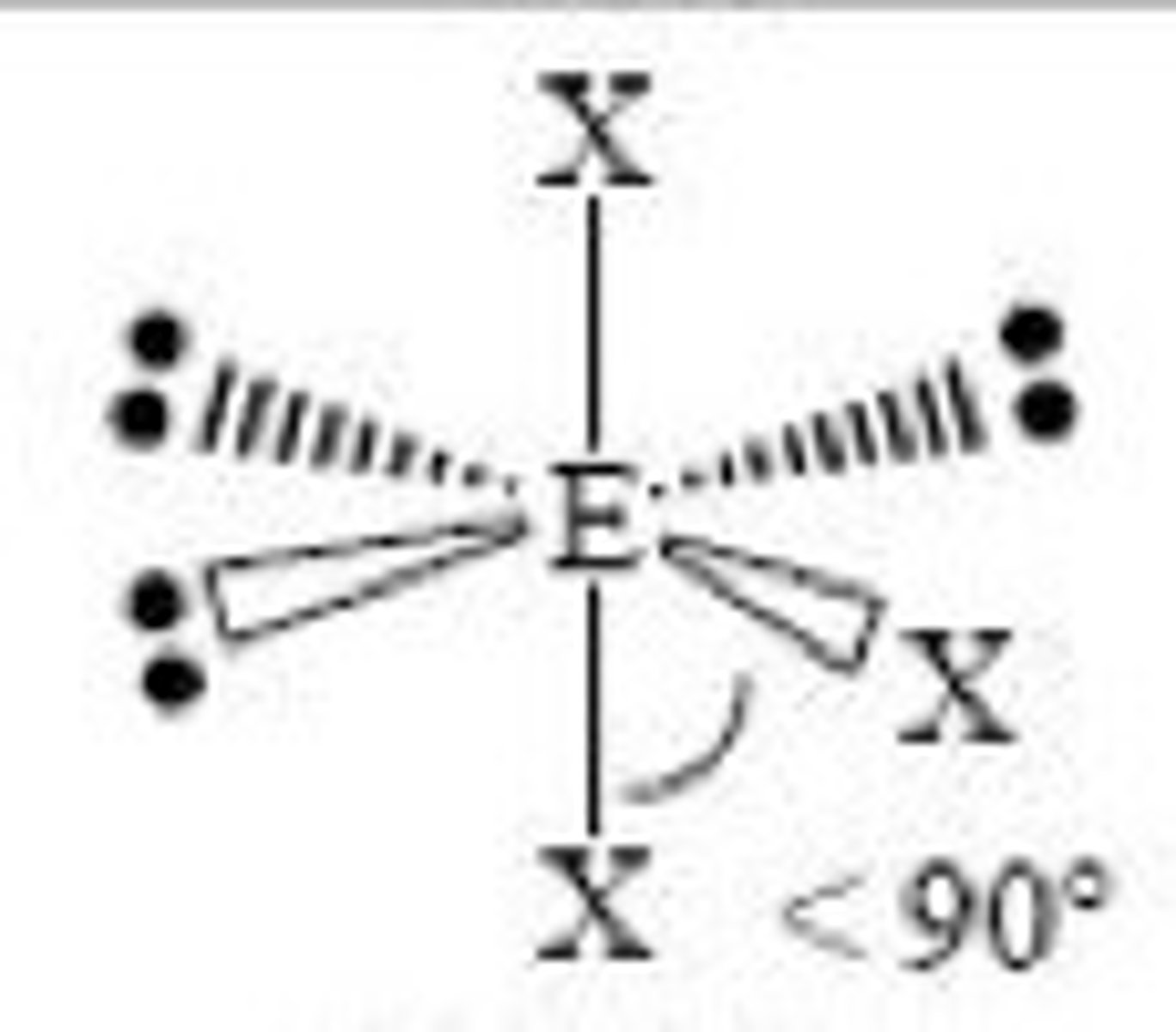
6 electron domains with 4 being lone pairs...
Molecular Geometry: Linear
180 degrees, nonpolar
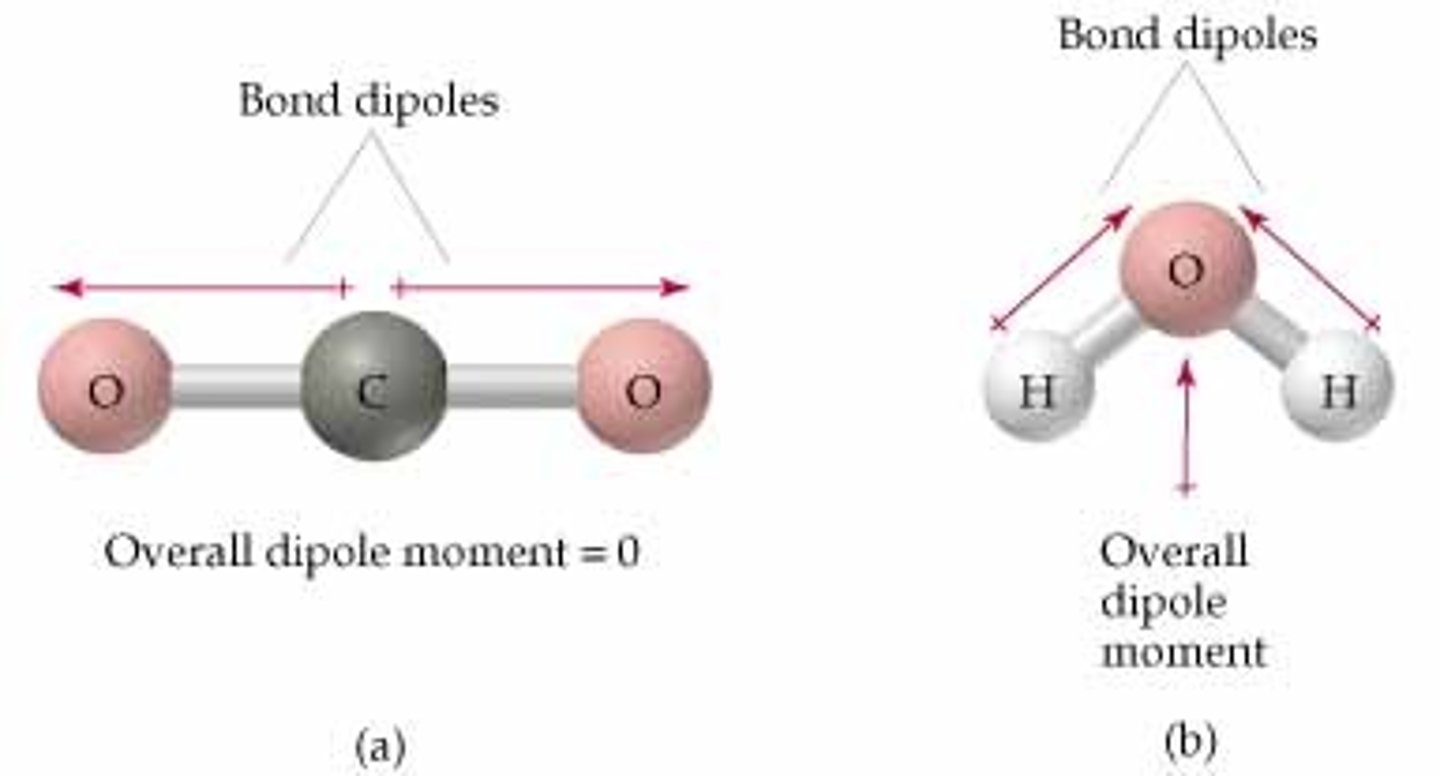
How to determine molecular polarity?
1. Molecule has polar bonds (ionic bonds and strong electronegative differences in covalent bonds greater than 0.4)
2. Unsymmetrical shape or unbalanced dipole vectors
3. If symmetrical shape but one terminal/bonding element is different from the others, then it is polar
How does polarity work?
Through the movements of electrons in covalent bonds, some regions become more partially negative and while others partially positive. This creates attractions between other polar molecules.
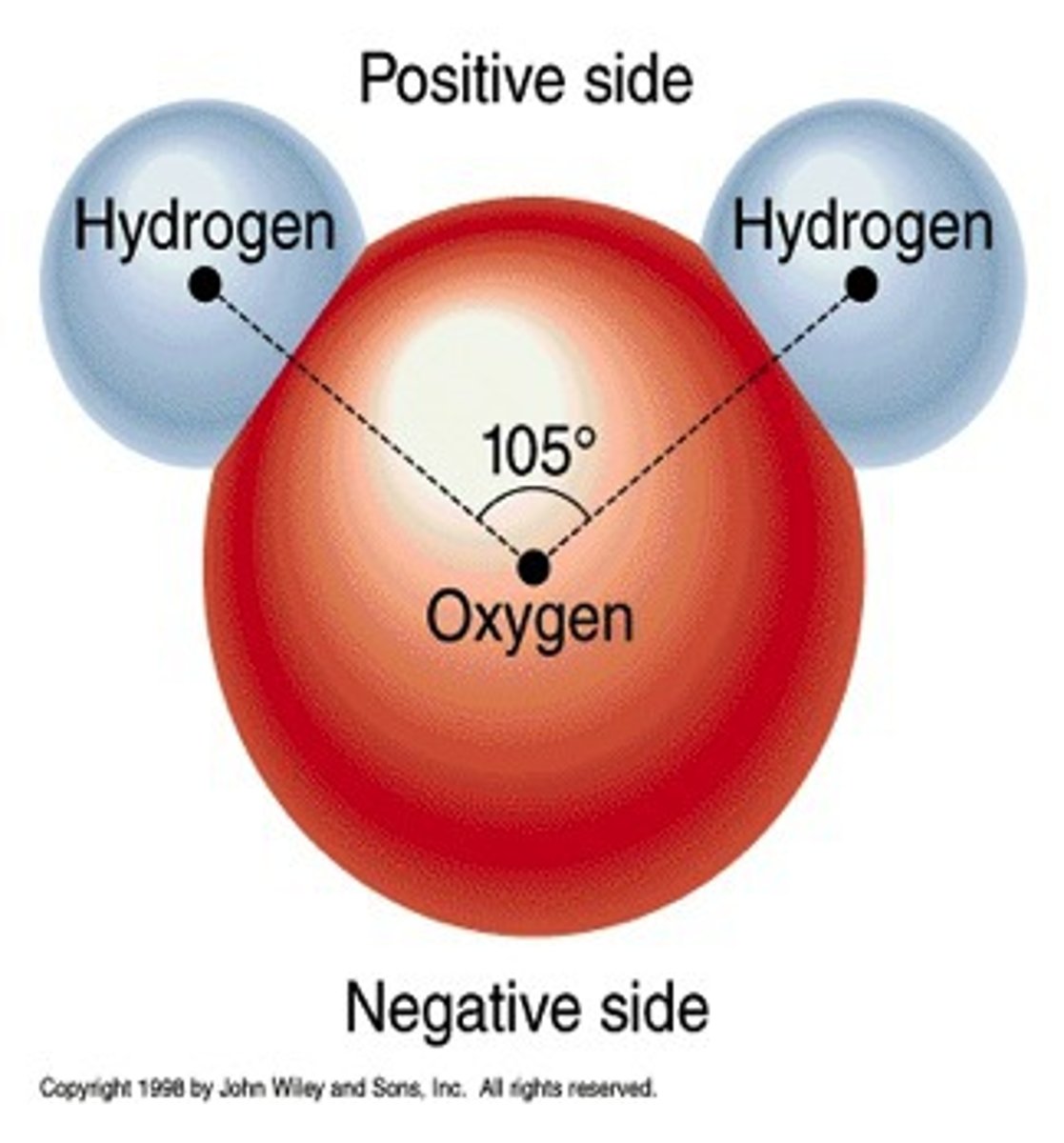
Dipole-dipole
A polar molecule's positive or negative region attracts the opposite partial charge of another polar molecule
3rd strongest intermolecular force (right below hydrogen-bonding)
Tip for remembering polarity through molecular geometries and bond angles... (assuming all terminal atoms are the same element)
Nonpolar shapes have exact angles (180, 120, 109.5, 90)
Polar shapes have deviating angles (less than or greater than 120, 109.5 90, etc.)
Intermolecular forces (secondary forces) are...
Attractive forces between molecules (and not the bonds, ionic or covalent, between elements inside molecule)
Much weaker than intramolecular forces (primary forces)
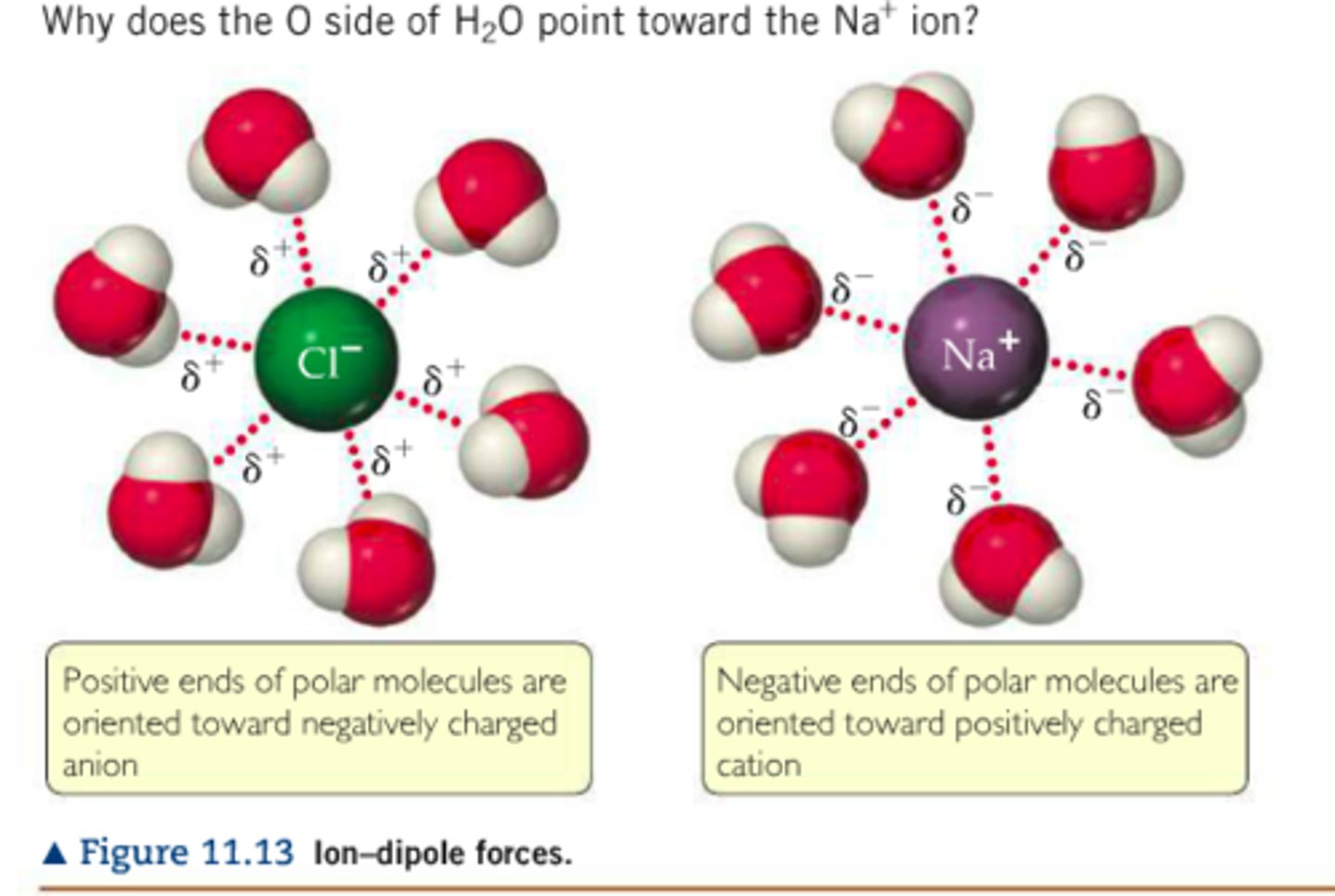
Ion-Dipole forces
An ion element (or polyatomic) attracting the opposite partial charge in a polar molecule (dipole is another word for polar molecule)
Strongest Intermolecular Force
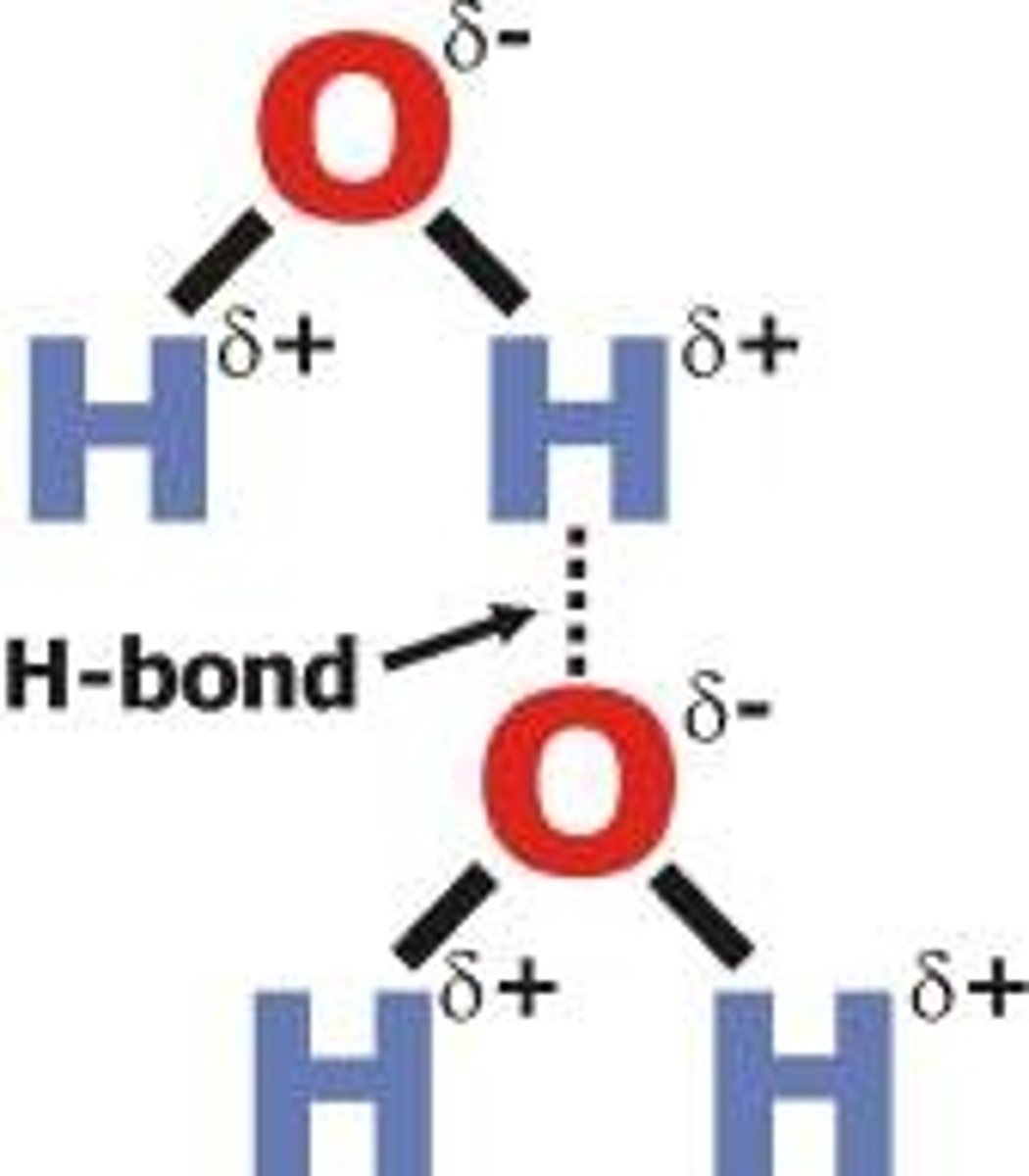
Hydrogen Bonding Force
When a hydrogen atom gets deshelled from a strong covalent bond (usually to N, O, or F) and has its proton attract other polar molecules
2nd strongest intermolecular force
London/Dispersion Forces
Temporary dipoles in atoms and molecules induced by other dipoles or ions (Ion-induced and dipole-induced)
(Can also occur between originally neutral atoms and molecules over time --> specifically London/dispersion force)
Weakest intermolecular force
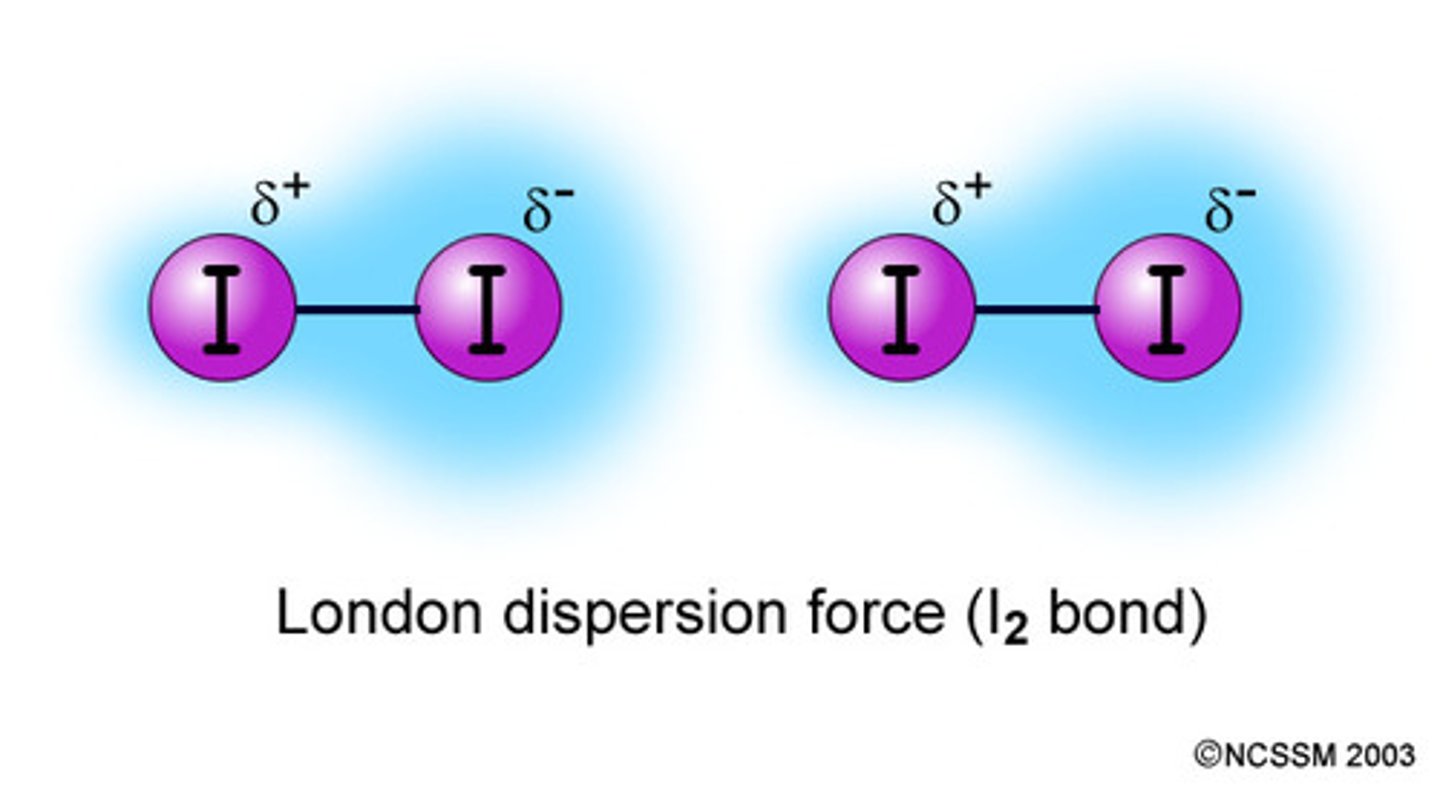
Van der Walls forces...
Category of intermolecular forces made of...
Hydrogen bonding and London/Dispersion forces
Relationship of boiling point of molecules to intermolecular forces...
More intermolecular forces in a molecule mean more energy (thus temperature) to cause a phase change
If a metal is part of the molecule, assume it takes the most energy
Larger molar mass = more electrons = larger electron cloud = increased polarizability = stronger attractions = higher boiling points
Larger molar mass = more electrons = larger electron cloud = increased polarizability = stronger attractions = higher boiling points
Does shape of a molecule affect intermolecular force (and boiling point)?
Yes, more surface area = more surface contact --> larger induced dipole --> stronger attractions
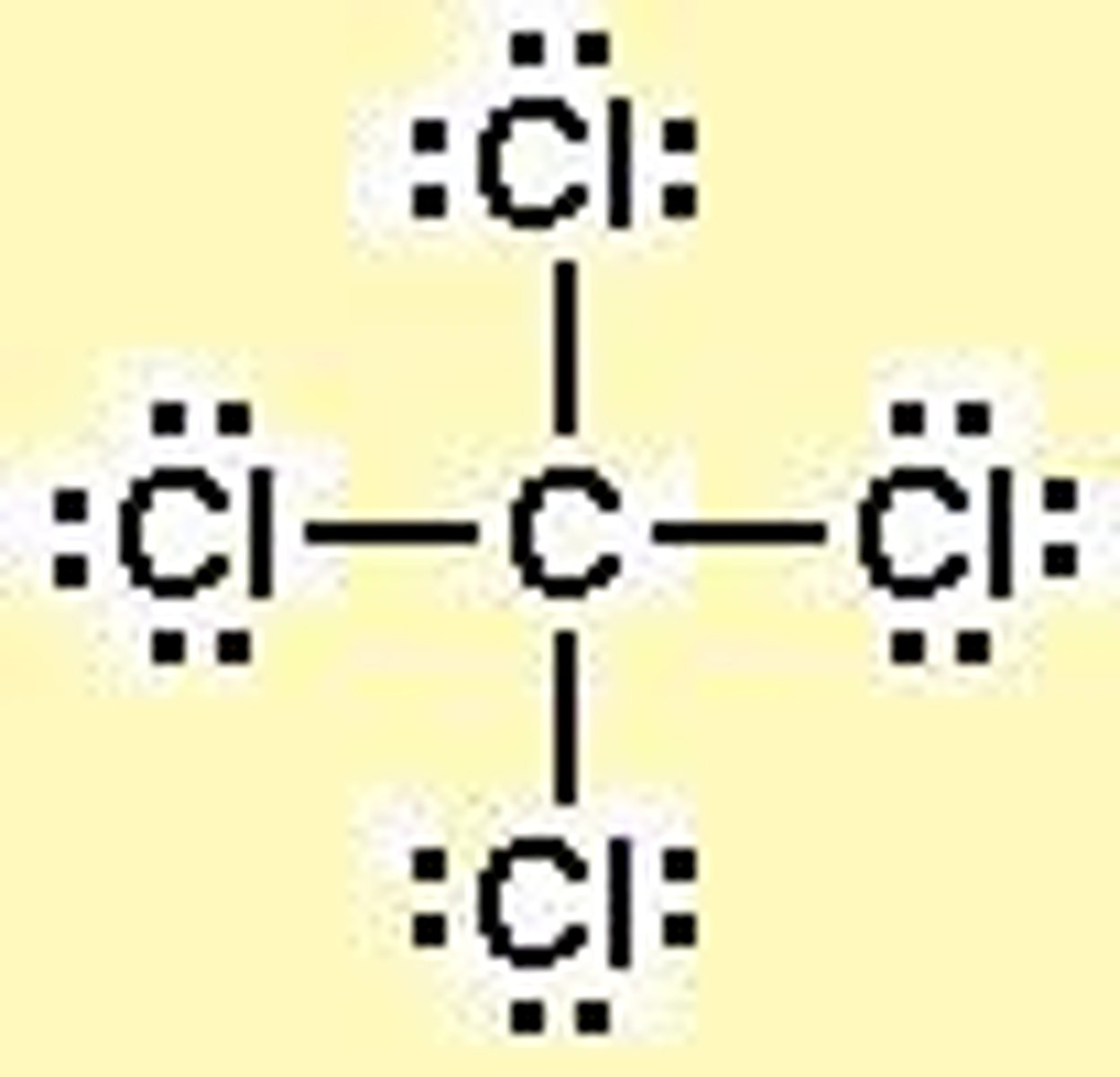
Practice: Determine CCl4's electron geometry, molecular geometry, polarity, and intermolecular forces.
EG: Tetrahedral
MG: Tetrahedral
Polarity: Nonpolar
IMFs: dispersion/london
Practice: What bond is C-C?
A pure covalent bond (same element)
Practice: What bond is N-F?
A polar bond (electronegative difference of 0.94)
Memorize: What bond is C-H?
A nonpolar bond (electronegative difference is less than 0.4)
Practice: Determine H2O's electron geometry, molecular geometry, polarity, and intermolecular forces.
EG: Tetrahedral
MG: Bent
Polarity: Polar
IMFs: dispersion/london, hydrogen bonding, and dipole-dipole
Practice: Determine NH3's electron geometry, molecular geometry, polarity, and intermolecular forces.
EG: Tetrahedral
MG: Trigonal pyramidal
Polarity: Polar
IMFs: dispersion/london, hydrogen bonding, and dipole-dipole
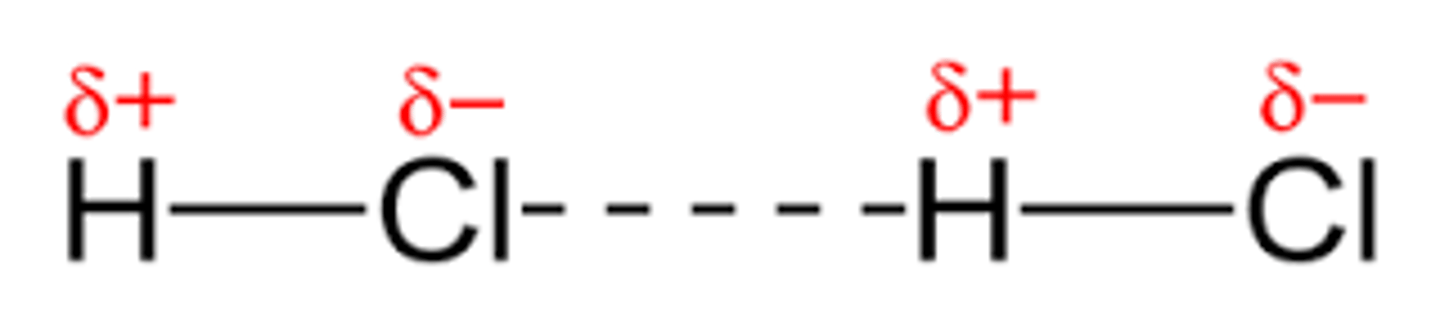
Practice: What bond is H-Cl and what intermolecular forces are included?
Polar bond with london/dispersion and dipole-dipole, but no hydrogen bonding (H must bond with N, O, or F)
IMF Strengths organized
Ion-dipole > Hydrogen-bonding > dipole-dipole > ion-induced dipole > dipole-induced dipole > dispersion/london
