CH 11 Properties of Liquids and Phase Changes: Intermolecular Forces and Phase Diagrams
1/103
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
104 Terms
What is the balance that determines the physical state of a substance?
Thermal energy (favors disorder) and interactions between molecules (favors order).
What are physical properties?
Properties that are readily observed and do not involve transformations into different substances.
What are chemical properties?
Properties that can only be determined when a substance is changed into another substance, involving the formation of new molecules.
What distinguishes intramolecular forces from intermolecular forces?
Intramolecular forces are strong forces that hold atoms together within molecules, while intermolecular forces are weak forces that act between molecules.
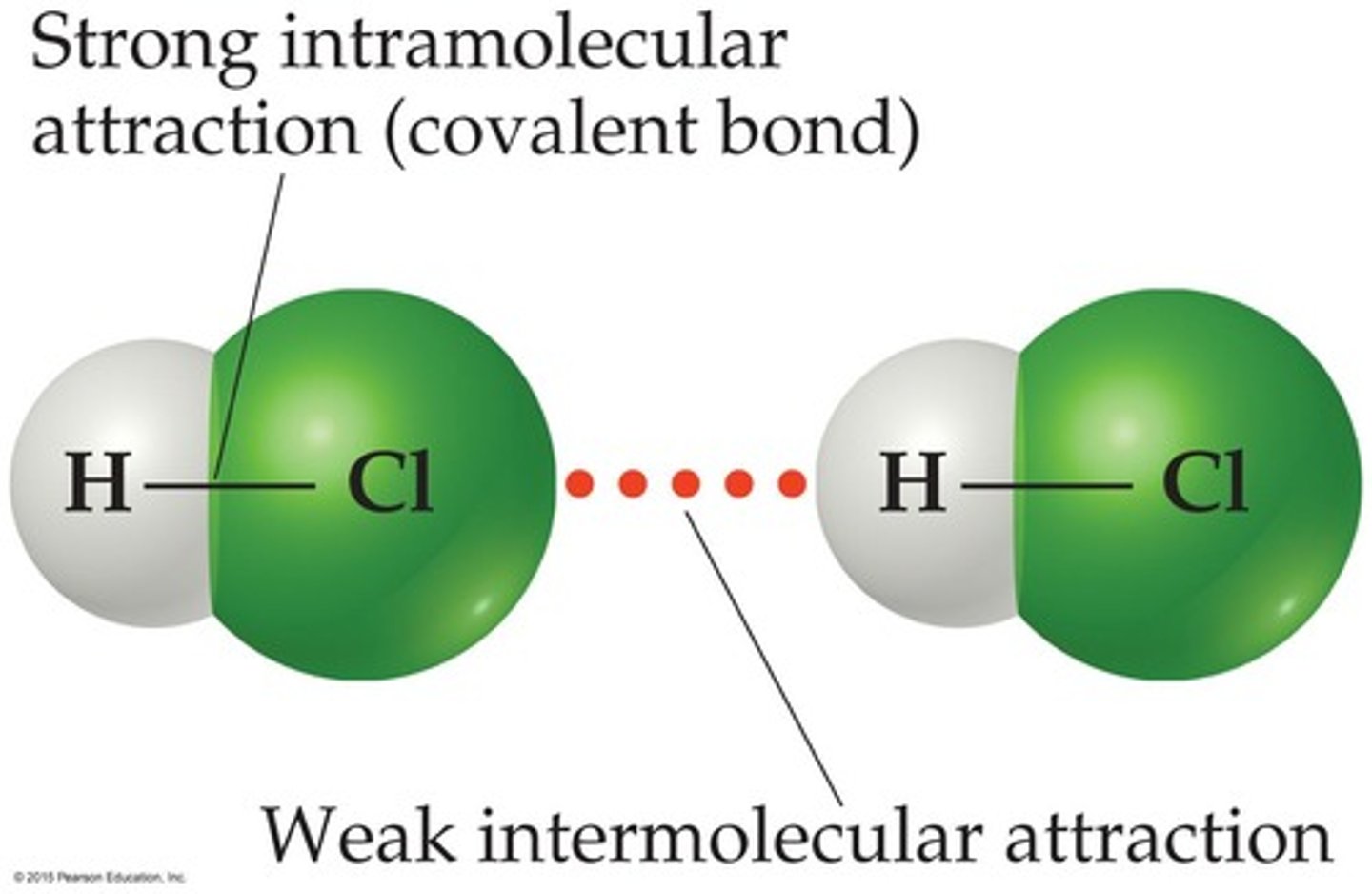
What determines the strength of intermolecular forces?
The strength depends on the magnitude of the electrostatic forces between molecules.
What is the relationship between intermolecular forces and physical properties?
Determines physical properties such as boiling point, melting point, and solubility.
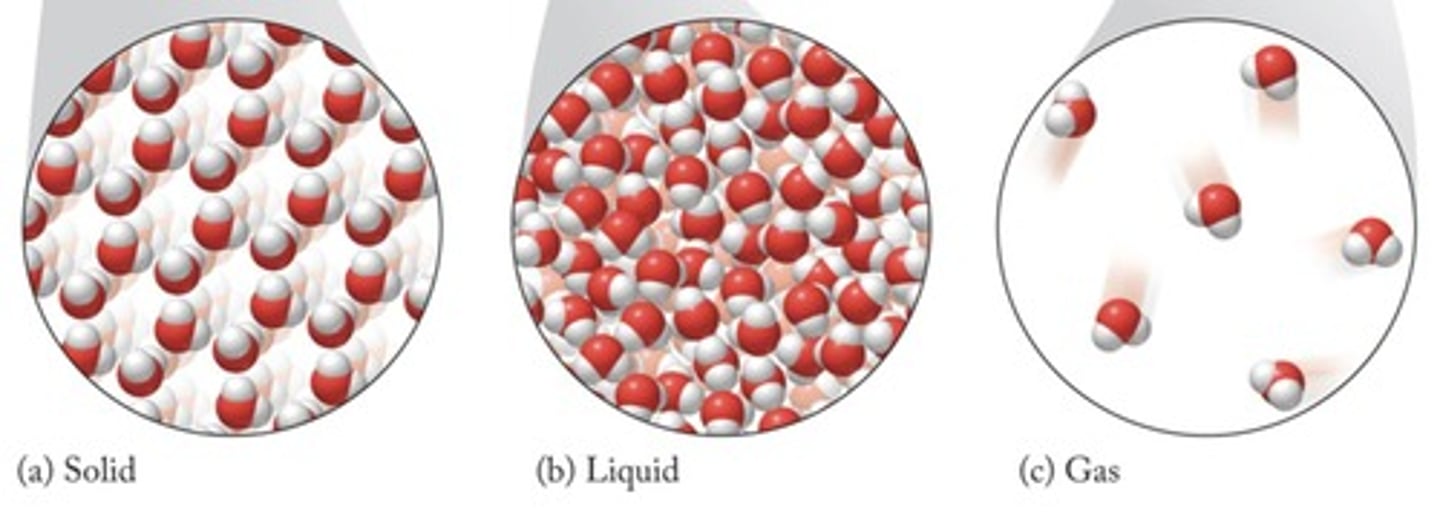
How does the physical state of a substance relate to its composition?
The physical state (solid, liquid, gas) is a physical property because the composition remains the same when a substance changes state.
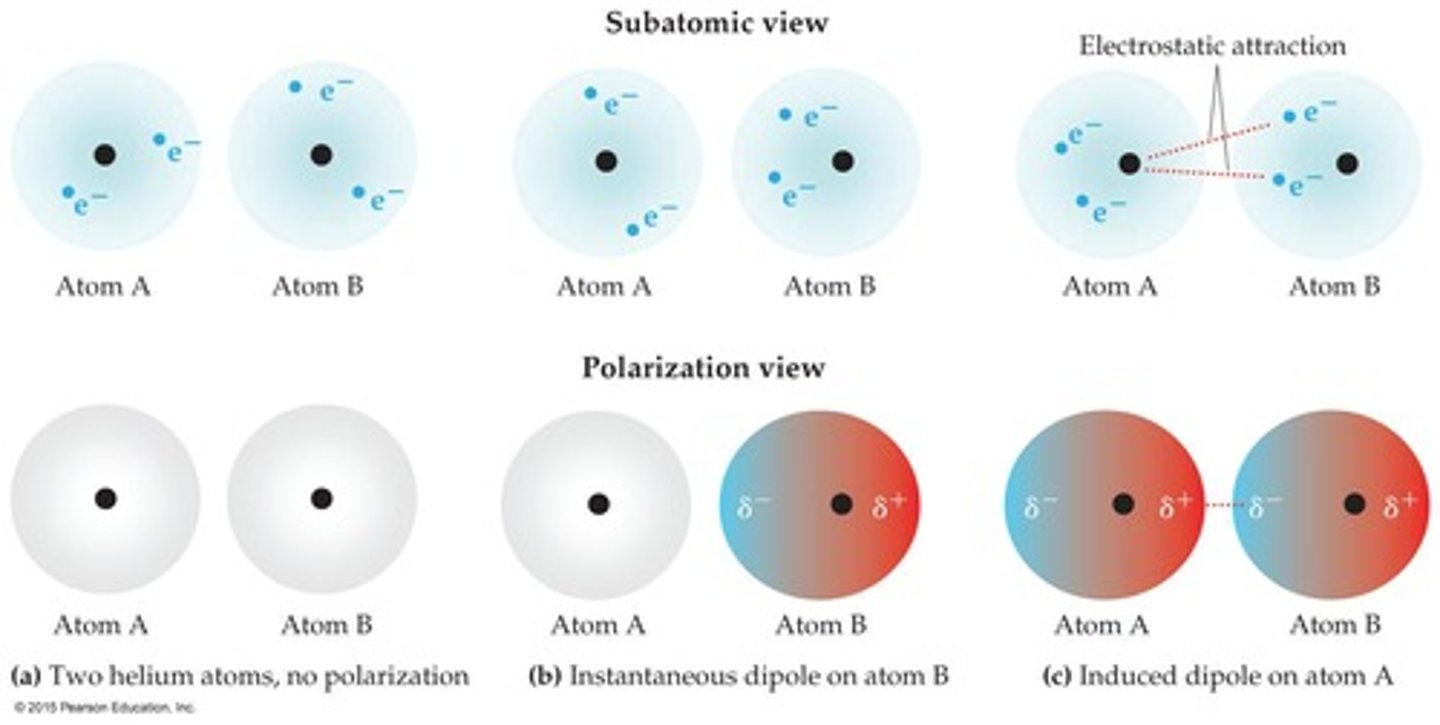
What is polarizability and how does it relate to dispersion forces?
Polarizability causes a temporary separation of charges, leading to dispersion forces, which are not dependent on polarity.
How do dispersion forces depend on molecular shape and size?
Dispersion forces are stronger with more contact/surface area; longer and/or larger molecules have stronger dispersion forces.
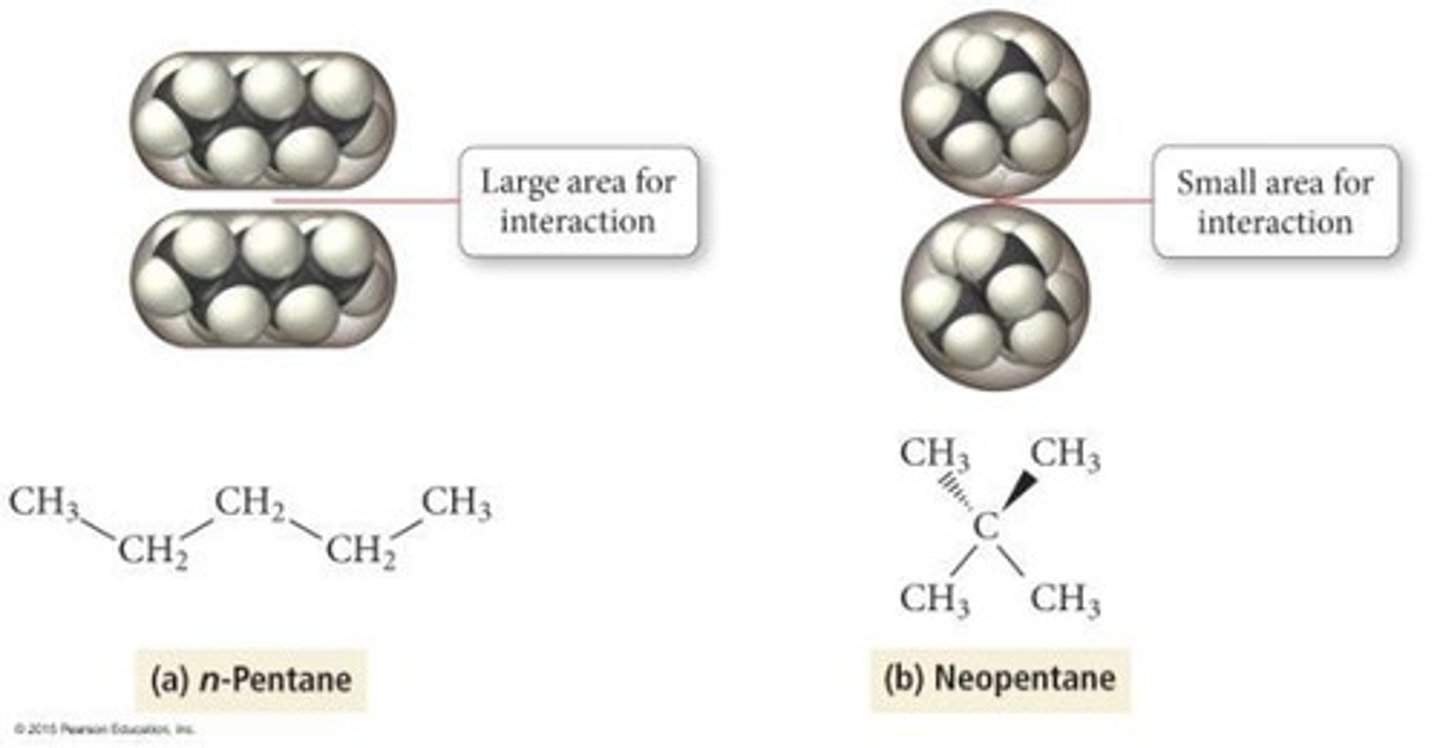
What trend is observed in the strength of dispersion forces with molecular size and mass?
Dispersion forces increase in strength with size and mass; larger molecules have stronger intermolecular forces.
When comparing two nonpolar molecules, which one has stronger intermolecular forces?
The heavier or larger nonpolar molecule has stronger dispersion forces.
How do polar and nonpolar molecules compare in terms of intermolecular forces?
The polar molecule usually has stronger forces, but dipole-dipole forces are generally more significant than dispersion forces.
What is the effect of attractive forces on boiling and melting points?
The stronger the attractive forces, the higher the boiling point and melting point.
What is the significance of phase changes in relation to physical properties?
Phase changes, such as boiling and melting, reflect changes in the interactions between molecules without altering their composition.
What is the difference between reversible and irreversible changes?
Reversible changes do not involve changing the molecules, while irreversible changes result in the formation of new substances.
What is the role of bonds in chemical reactivity?
Bonds (intramolecular forces) determine the chemical reactivity of substances.
What is the relationship between thermal energy and molecular interactions?
Thermal energy favors disorder, while interactions between molecules favor order.
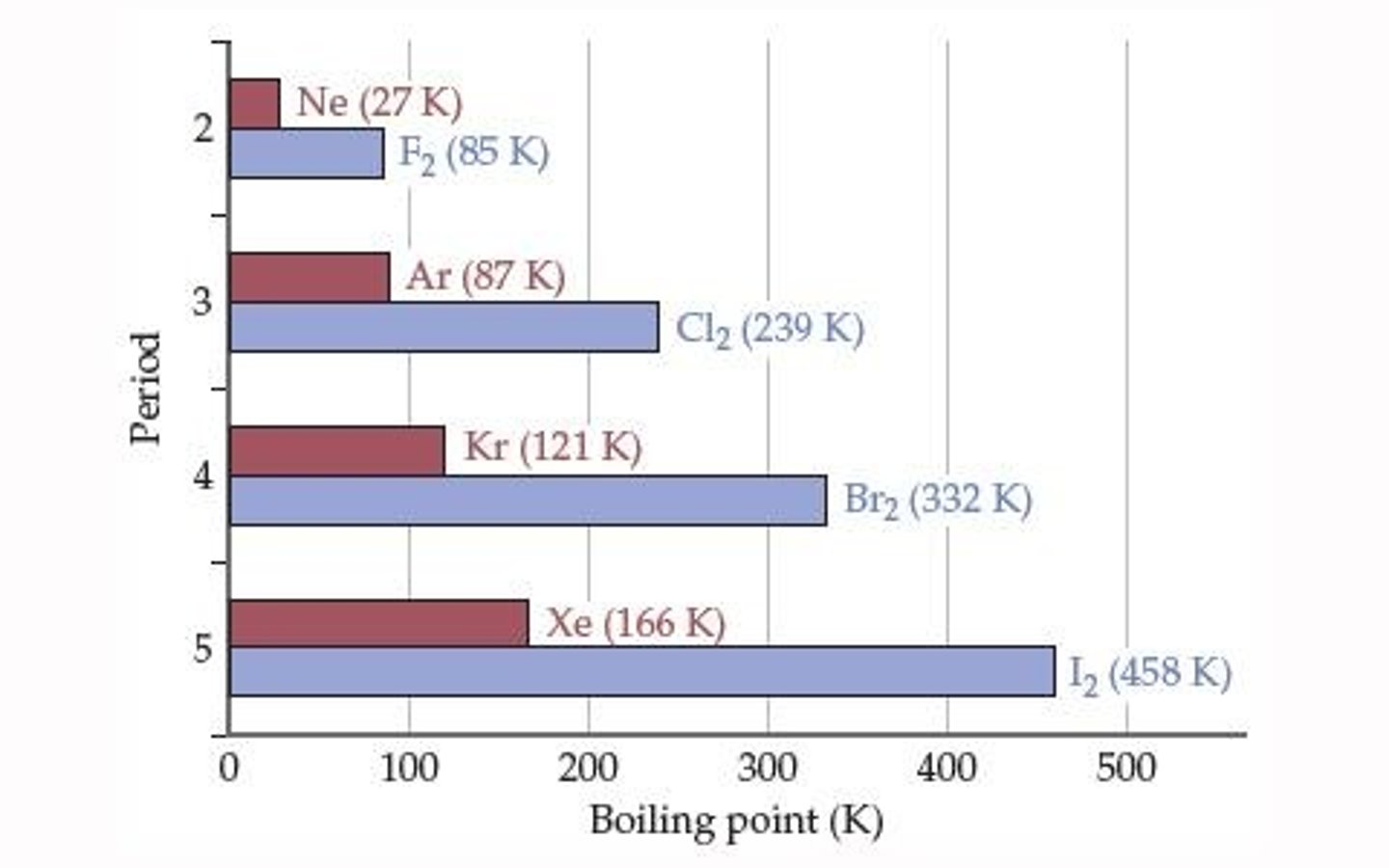
Why is the boiling point of halogens greater than that of noble gases in the same period?
The boiling point of halogens is greater due to stronger intermolecular forces compared to the weak dispersion forces in noble gases.
What are the two main types of forces that affect molecular interactions?
Intramolecular forces (within molecules) and intermolecular forces (between molecules).
What factors influence solubility in mixtures versus pure substances?
Solubility is influenced by the nature of intermolecular forces in mixtures compared to those in pure substances.
What are halogens and their characteristics?
Halogens are diatomic molecules that are slightly polar, have greater mass and size.
What interactions occur between polar molecules?
Dipole-dipole interactions occur between polar molecules.
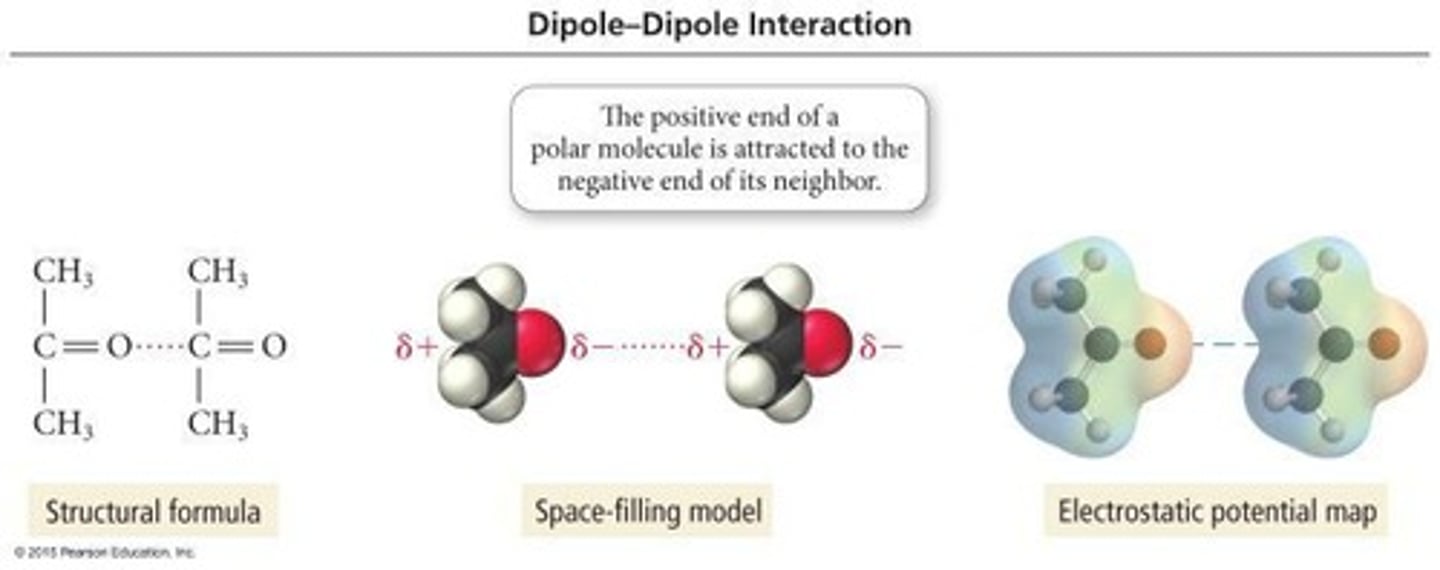
What determines if a molecule has dipole-dipole forces?
A molecule has dipole-dipole forces if it is polar, meaning it has differences in electronegativity.
What is electronegativity?
The ability of an atom to attract electrons within a bond.
How does molecular shape affect molecular polarity?
Molecules are polar if they have polar bonds and the shape does not cancel out the bond dipoles.
What are the general guidelines for determining if a molecule is polar or nonpolar?
A molecule is nonpolar if it has no polar bonds or all identical bonds with no lone pairs. It is polar if it has polar bonds and either lone pairs on the central atom or different types of bonds.
What are hydrogen bonds?
Exceptionally strong dipole-dipole interactions that occur between N, O, or F and H.
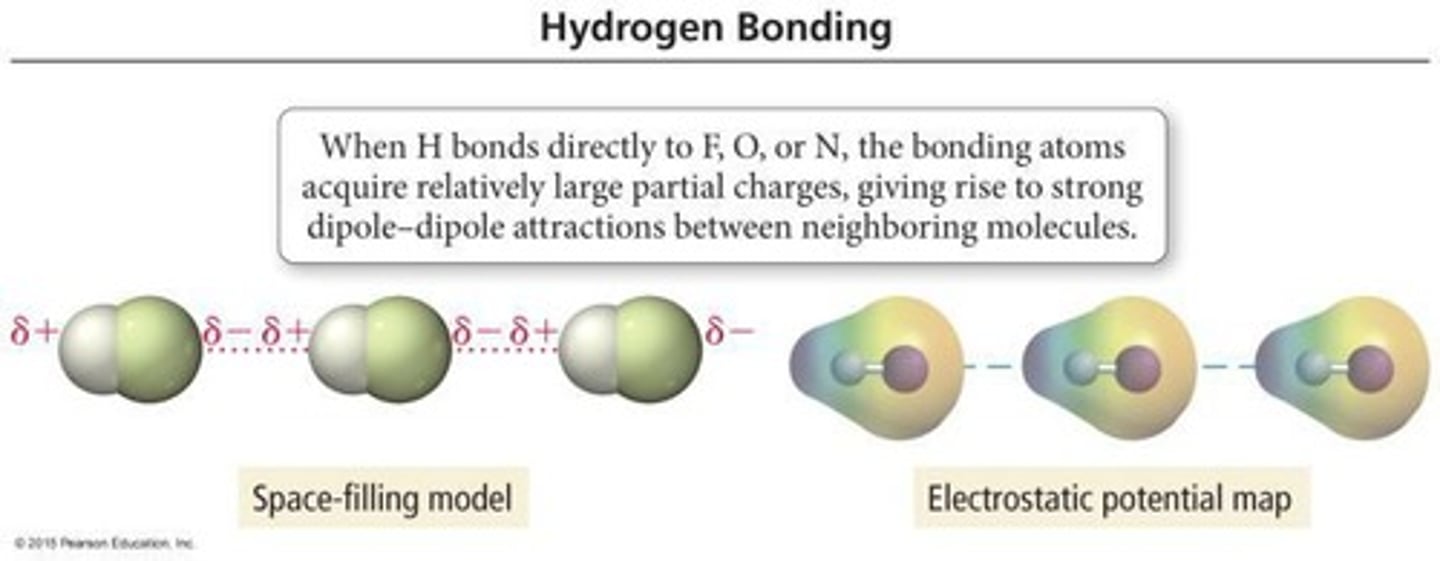
What is the strongest intermolecular force in ethanol (CH3CH2OH)?
Hydrogen bonding, dipole-dipole, and dispersion forces.
Which molecule has only dispersion forces?
O2 has only dispersion forces.
What kind of intermolecular forces must be overcome when water boils?
Dispersion forces, dipole-dipole forces, and hydrogen bonds must be overcome when water boils.
What kind of intermolecular forces must be overcome when ice melts?
Hydrogen bonds must be overcome when ice melts.
What is a characteristic of molecules with hydrogen bonding?
They have N-H or O-H bonds, which create strong dipole-dipole forces.
What is the relationship between molecular weight and boiling point?
Generally, larger or heavier molecules have stronger dispersion forces, affecting their boiling points.
What is the effect of lone pairs on molecular polarity?
Lone pairs on the central atom can contribute to a molecule being polar.
What is the significance of molecular asymmetry in polarity?
Asymmetrical molecules with polar bonds are usually polar because the bond dipoles do not cancel out.
How do dipole-dipole forces compare to dispersion forces?
Dipole-dipole forces are generally stronger than dispersion forces.
What is required for a bond to be considered polar?
If the bonds are between atoms with different electronegativities.
What intermolecular forces are present in dimethyl ether (CH3OCH3)?
dipole-dipole and dispersion forces.
Which molecule should have the highest boiling point: H2, N2, CO, HF?
HF should have the highest boiling point due to hydrogen bonding.
Which molecule should have the lowest boiling point: CH4, CBr4, SiH4, CF4?
CH4 should have the lowest boiling point.
What is the role of hydrogen bonds in determining boiling points?
Hydrogen bonds significantly increase boiling points compared to other intermolecular forces.
What are the types of intermolecular forces?
Dispersion forces, dipole-dipole forces, and hydrogen bonds.
How do intermolecular forces relate to physical properties?
The strength of intermolecular forces affects properties such as boiling and freezing points, surface tension, viscosity, and capillary action.
What is the relationship between intermolecular force strength and boiling/melting points?
Stronger intermolecular forces lead to higher boiling and melting points, requiring more energy to break apart molecules.
What is surface tension and how is it affected by intermolecular forces?
The tendency of liquids to minimize surface area; stronger intermolecular forces result in greater surface tension.
What causes the 'beading' of liquid drops?
Caused by cohesive forces within the liquid that create surface tension.
What are adhesive forces and how do they affect wetting?
Interactions between the liquid and a surface; strong adhesive forces promote wetting, while weak adhesive forces lead to beading.
What is the difference between cohesive and adhesive forces in the context of a meniscus?
A convex meniscus forms when cohesive forces are greater than adhesive forces, while a concave meniscus forms when adhesive forces are greater.
What is capillary action?
the ability of a liquid to climb upwards through a narrow tube, influenced by adhesive and cohesive forces.
Which factors counteract capillary action?
Cohesive forces and gravity counteract capillary action.
What happens to the height of water in a capillary tube if the diameter is increased?
Increasing the diameter of the tube leads to a smaller height of water due to reduced adhesive forces relative to cohesive forces.
What are the phase changes associated with solid and liquid states?
Melting (fusion) and freezing are the phase changes
What are the phase changes associated with liquid and gas states?
Vaporization (boiling) and condensation are the phase changes
What is the relationship between energy and phase changes?
Endothermic phase changes require heat, while exothermic phase changes release heat.
What happens to temperature during melting or freezing?
The temperature remains constant during melting or freezing as the solid and liquid are in equilibrium.
How is the heat required to melt a substance calculated?
The heat required to melt a substance is calculated using the formula q = n•∆Hfus.
What is the value of ∆Hfus for H2O at 0°C?
∆Hfus for H2O at 0°C is 6.02 kJ/mol.
How much heat is required to melt 100 g of ice at 0°C?
To calculate the heat required, convert 100 g of ice to moles (100 g / 18.02 g/mol) and multiply by ∆Hfus (6.02 kJ/mol).
What is the significance of enthalpies (∆H) in phase changes?
Enthalpies are equal but have opposite signs for endothermic and exothermic phase changes.
What role do intermolecular forces play in the properties of liquids?
These forces determine properties such as boiling point, surface tension, and viscosity.
What is the effect of temperature on surface tension?
Higher temperatures result in weaker surface tension due to increased molecular motion.
What type of surfaces promote strong adhesive forces with water?
Polar surfaces promote strong adhesive forces with liquids that have dipole or hydrogen bonding.
What is the heat of fusion (∆Hfus) for H2O(s) at 0°C?
6.02 kJ/mol
What is the equation for calculating heat during a phase change from liquid to gas?
q = n•∆Hvap
What is the relationship between ∆Hvap and ΔHcondensation?
∆Hvap = -ΔHcondensation
What happens to the temperature of a liquid during boiling?
The temperature remains constant until all the liquid has boiled off.
What is the formula for calculating heat changes within a single phase?
q = m•Cs•∆T, where m is mass, Cs is specific heat, and ∆T is the change in temperature.
What is the specific heat (Cs) value for liquid water (H2O)?
4.18 J/g•°C
How do you calculate the heat required to heat 100 g of water from 0°C to 25°C?
Using q = m•Cs•∆T: q = 100 g • 4.18 J/g•°C • (25°C - 0°C).
What is the heat required to melt 100 g of ice at 0°C?
q = n•∆Hfus, where n is the number of moles of ice.
What is the heat required to boil off all the resulting water after melting 100 g of ice?
q = n•∆Hvap, where ∆Hvap = 40.7 kJ/mol for H2O(l) at 100°C.
What is the difference between boiling and evaporation?
Boiling occurs at a fixed temperature with heat input, while evaporation occurs below the boiling point without heat input.
What factors increase the rate of evaporation?
Higher temperature and larger surface area.
What is vapor pressure?
The pressure of evaporated gas molecules above a liquid.
What is volatility in the context of liquids?
Refers to how easily a liquid evaporates.
How do intermolecular (IM) forces affect volatility?
Weaker IM forces result in higher volatility and higher vapor pressure.
What is the effect of temperature on vapor pressure?
Vapor pressure increases with higher temperature.
What is the relationship between vapor pressure and boiling point?
A liquid with higher vapor pressure has a lower boiling point.
What happens to the rate of evaporation at higher pressure?
The rate of evaporation decreases at higher pressure.
What is the equation for heat during phase transitions?
q = n•∆Hfus for melting and q = n•∆Hvap for boiling.
What is the significance of the boiling point?
It is the fixed temperature at which the liquid and gas are in equilibrium.
What is the formula for calculating the heat required to heat a substance through a temperature change?
q = m•Cs•∆T.
What is the effect of surface area on the rate of evaporation?
The rate of evaporation increases with larger surface area.
What is the chemical formula for water?
H2O
What is the chemical formula for pentane?
CH3CH2CH2CH2CH3
What is the chemical formula for isopropyl alcohol?
CH3CH(OH)CH3
What is the chemical formula for acetone?
CH3C(O)CH3
What is the normal boiling point defined as?
The temperature at which the vapor pressure equals 1 atm.
How does vapor pressure change with temperature?
Vapor pressure increases with temperature.
When does a liquid boil?
when its vapor pressure equals the applied atmospheric pressure.
How does atmospheric pressure affect boiling points?
At higher pressure, boiling points increase; at lower pressure, boiling points decrease.
What is the relationship between intermolecular forces and phase change energies?
Substances with greater intermolecular forces have larger ∆Hfus and ∆Hvap.
What is the relationship between ∆Hfus and ∆Hvap?
∆Hfus is less than ∆Hvap.
What is the equation that relates the enthalpy of sublimation to fusion and vaporization?
∆Hsub = ∆Hfus + ∆Hvap.
What does the Clausius-Clapeyron equation describe?
It describes how vapor pressure increases exponentially with temperature.
What is the slope of the plot of Pvap vs (1/T) according to the Clausius-Clapeyron equation?
The slope is -∆Hvap/R.
How can you use the Clausius-Clapeyron equation to find vapor pressure at different temperatures?
If you know ∆Hvap and Pvap at one temperature, you can solve for Pvap at another temperature.
What is the significance of the triple point in a phase diagram?
At the triple point, solids, liquids, and gases are all in equilibrium.
What happens to the phases of a substance below the triple point?
Below the triple point, liquids cannot exist.
What defines a supercritical fluid?
Exists above the critical point, where it is too dense to be a gas and too random to be a liquid.