Topic 2: Inorganic Chemistry II - Crystal Field Theory
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Crystal Field Theory (CFT)
A model describing how the d-orbitals of transition metal ions split in energy when surrounded by ligands in a complex.
Ligand Field Theory
An advanced version of MO Theory applied to transition metal complexes (d-orbital electrons that exceed the octet rule) that explains orbital interactions and symmetry in detail.
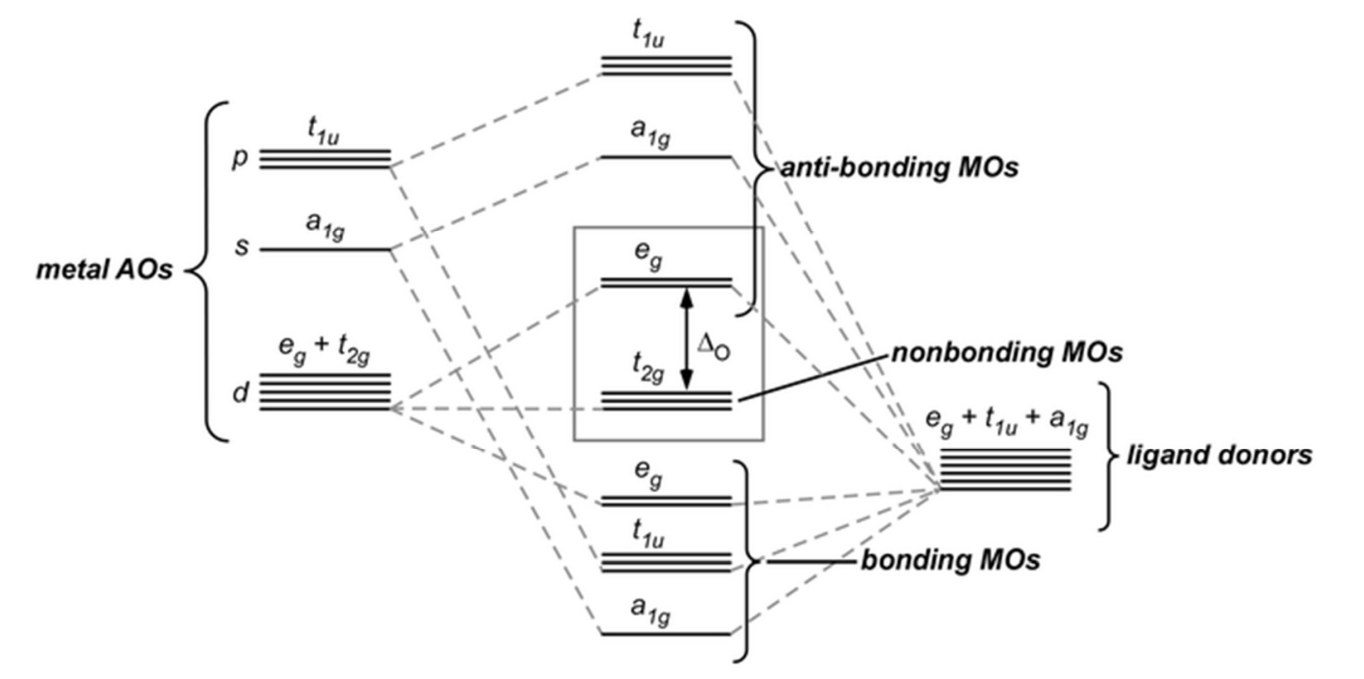
Crystal Field Theory (CFT) vs. Molecular Orbital Theory (MO)
CFT focuses only on d-orbitals and their interactions with ligands, ignoring full MO complexity.
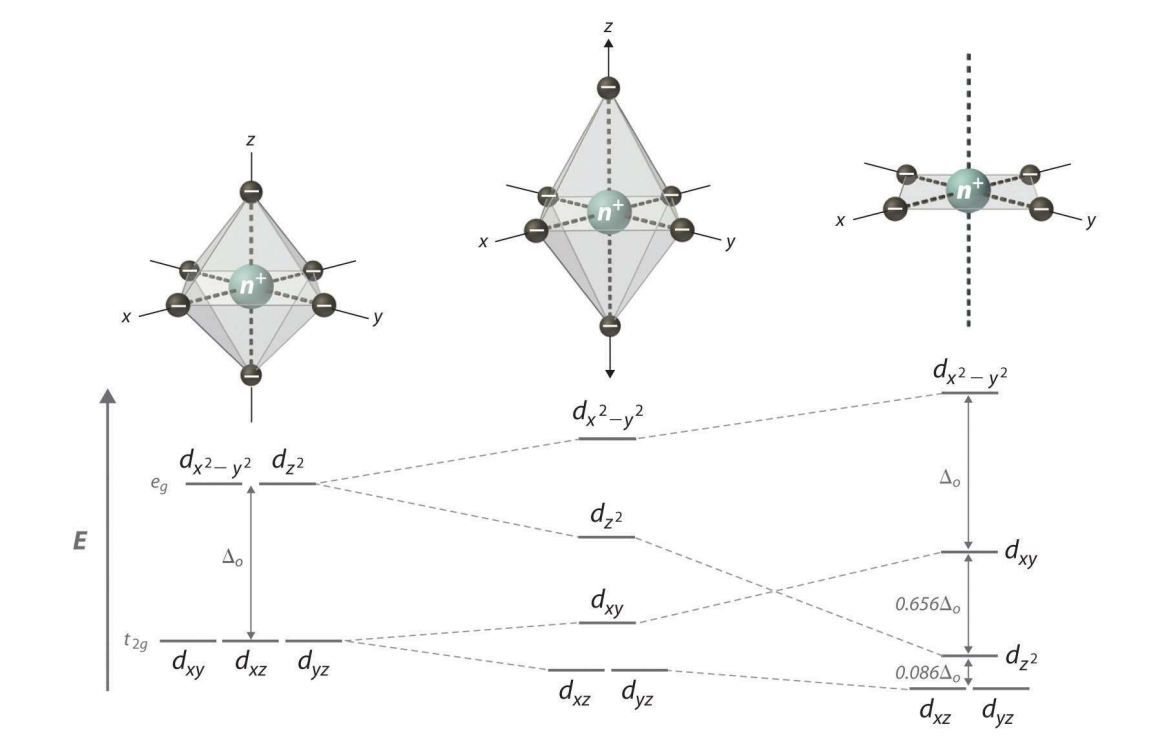
Formation of Transition Metal Complexes - Octahedral Geometry
Most TM compounds adopt an octahedral geometry with six ligands about the metal center, positioned along the Cartesian axes (±X, ±Y, ±Z).
Attraction: Highly stable due to attraction between negative ligand charges and the positive metal cation.
Repulsion: Ligand lone pairs act as negative point charges that also repel the negatively charged d-electrons of the metal, creating local electrostatic repulsion.
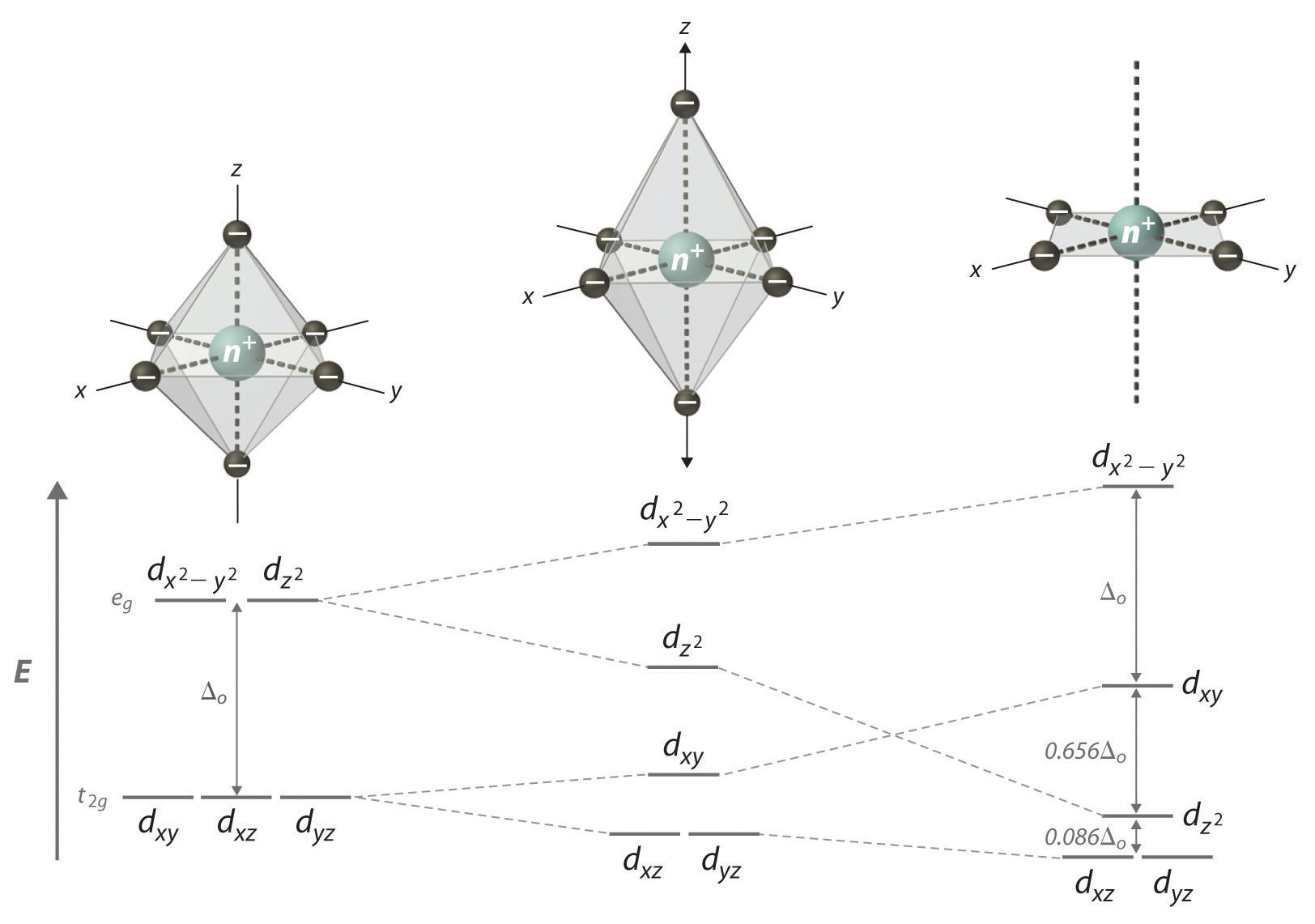
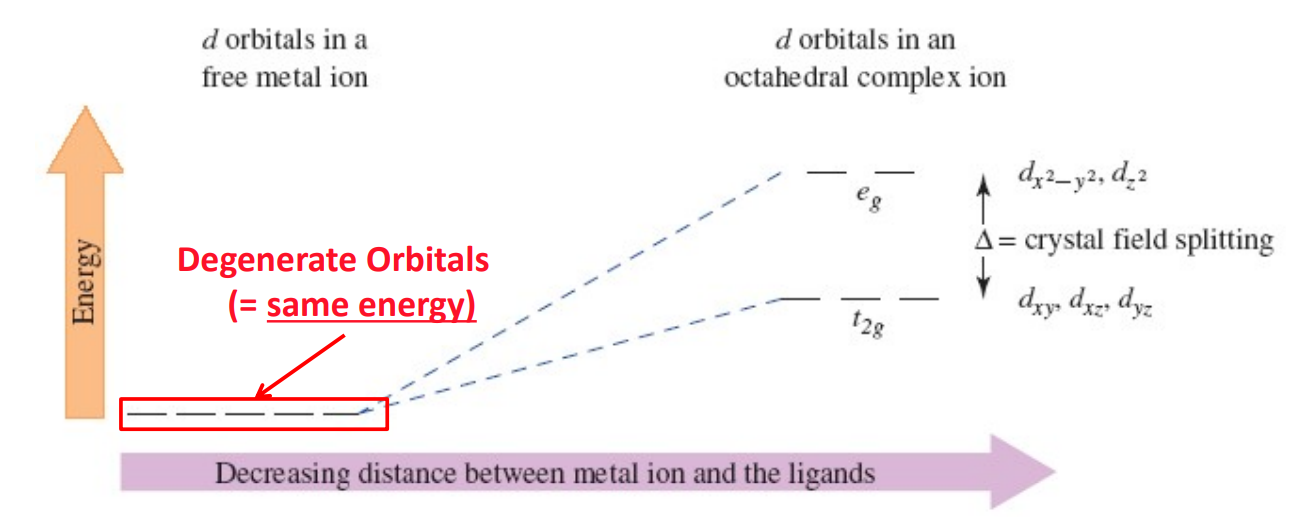
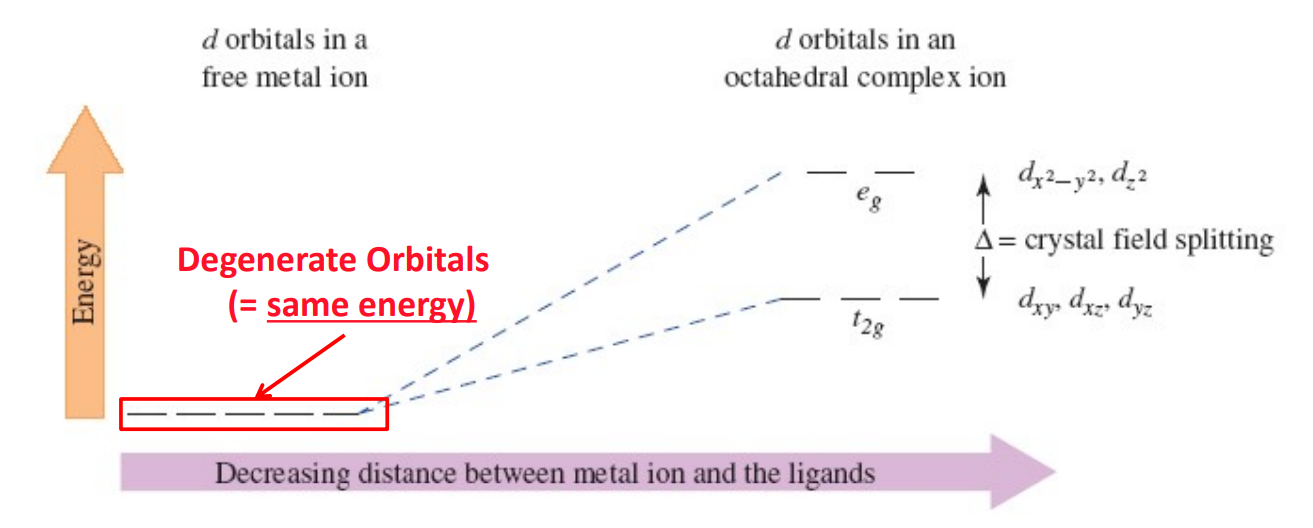
Formation of Transition Metal Complexes - Degeneracy of d-Orbitals and Splitting
In a free ion, all five d-orbitals have equal energy. The approach of ligands removes this degeneracy due to ligand electron lone pair to metal center d-electrons repulsion, causing orbital splitting.
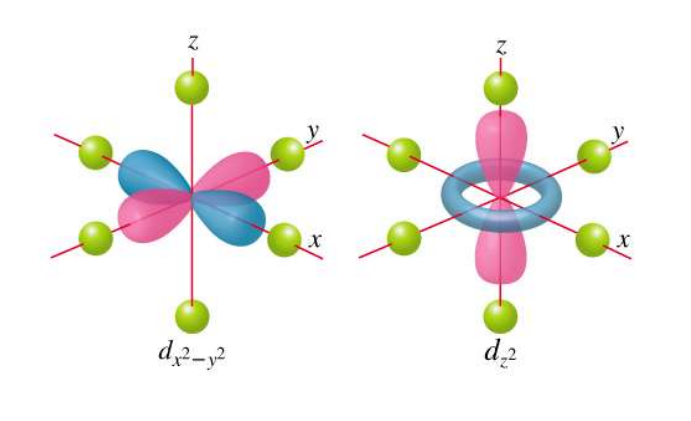
eg Orbitals
dₓ²₋ᵧ² and dz² orbitals
Point directly toward ligands and are destabilized more strongly (higher energy).
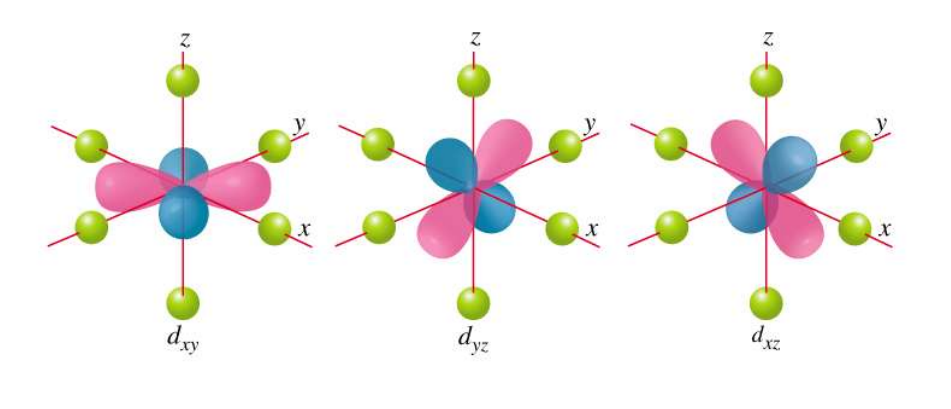
t2g Orbitals
dxy, dxz, dyz orbitals
Point between ligands and are less destabilized (lower energy).
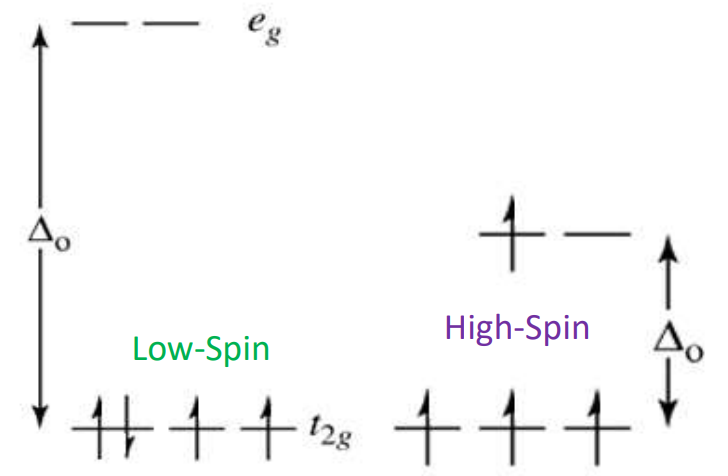
Crystal Field Splitting Energy (Δₒ)
The energy gap between t2g and eg orbitals caused by ligand interactions in an octahedral field.
Magnitude of Δₒ
Depends on the metal, its oxidation state, the ligands, and the geometry of the complex.
Effect of Oxidation State on Δₒ
Higher oxidation states of the TM cause larger Δₒ due to stronger ligand-metal interactions.
Periodic Trend in Δₒ
Increases down a group
2nd (Y to Ag) and 3rd (La to Au) transition series have larger splitting, leading to more low-spin complexes.
Spectrochemical Series
I⁻ < Br⁻ < Cl⁻, SCN⁻ < F⁻ < OH⁻ < ox²⁻ < H₂O < NH₃ < en < NO₂⁻ < CN⁻, CO
Ranks ligands from weak to strong field.
Where: ox²⁻ = oxalate and en = ethylenediamine
Weak-Field Ligands
Halides and O-donors (I⁻ to H₂O) — small Δₒ.
Strong-Field Ligands
N-donors and C-donors (NH₃ to CO) — large Δₒ.
Electron Filling in CFT - d-Electron Configuration
Only the d-electrons of the metal ion are considered for filling split orbitals (e.g., Fe²⁺ is d⁶).
Pairing Energy (P)
Energy required to pair two electrons in the same orbital.
Since electrons repel each other, it costs energy to pair them in the same orbital.
Competes with Δₒ to determine spin state.
Low-Spin complex (Δₒ > P)
Electrons pair in t2g before entering eg
Formed by strong-field ligands
Leaves few unpaired electrons.
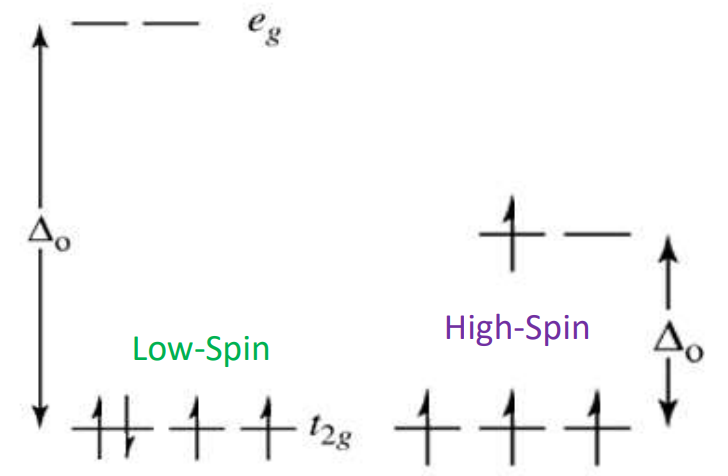
High-Spin Complex (Δₒ < P)
Electrons occupy all orbitals singly before pairing
Formed by weak-field ligands
Many unpaired electrons.
Electron Configurations Affected by Spin
d⁴, d⁵, d⁶, and d⁷ systems can be high or low spin depending on Δₒ and P.
Electron Configurations Unaffected by Spin
d⁸, d⁹, d¹⁰ systems have fixed filling regardless of Δₒ magnitude.
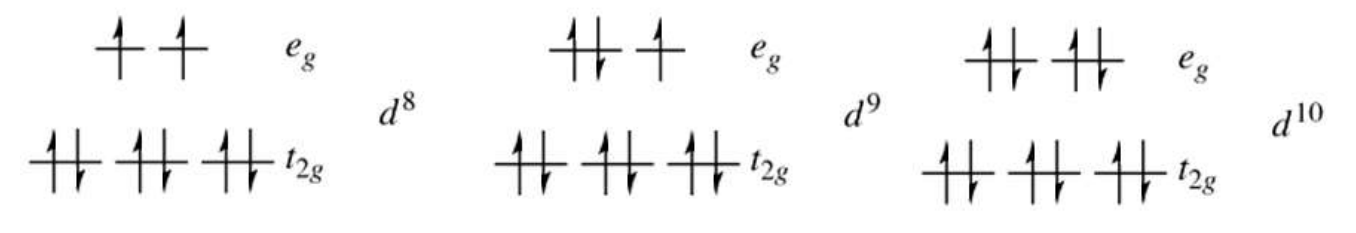
Magnetic Properties
Depend on the number of unpaired electrons
Paramagnetic (unpaired → high-spin complexes)
Diamagnetic (all paired → low-spin complexes)
Non-Octahedral Geometries
Other shapes (square planar, tetrahedral, etc.) cause different d-orbital splitting patterns.
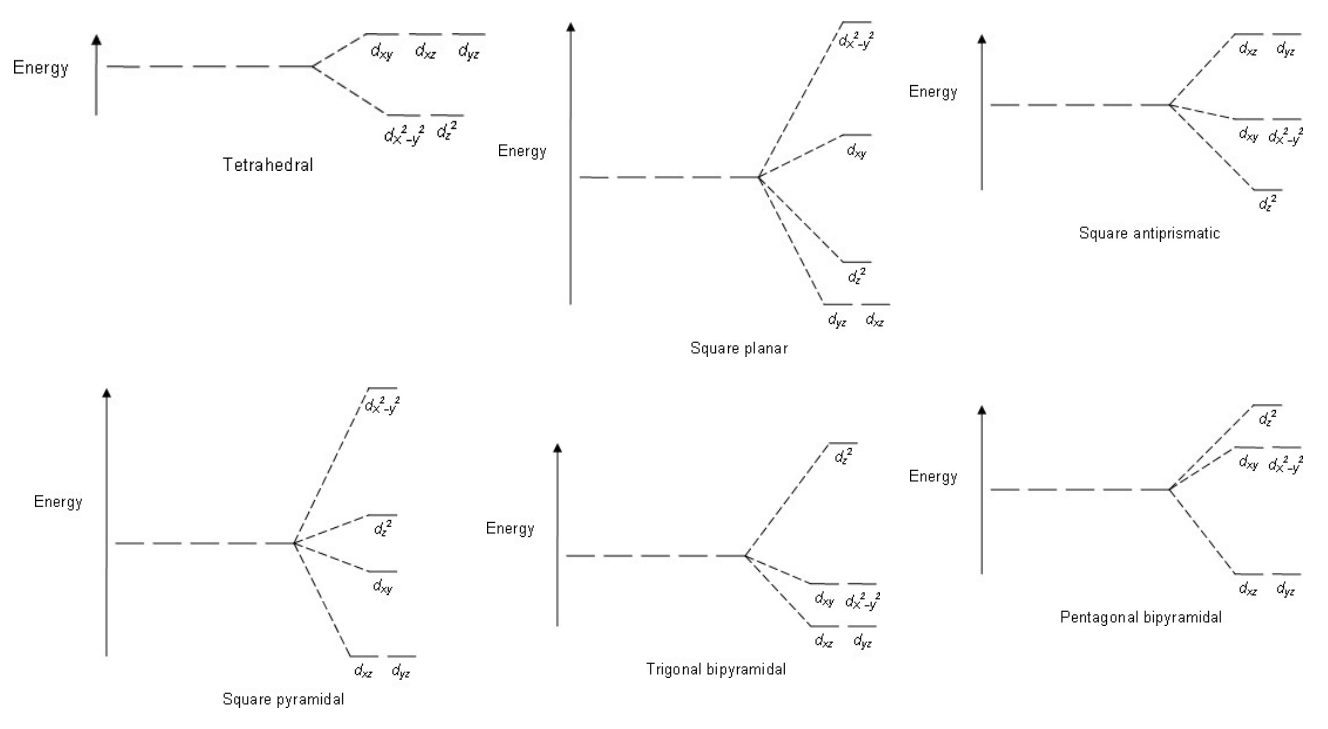
Tetrahedral Geometry
Produces an inverted splitting pattern compared to octahedral (t2g orbitals higher than eg).
Formation of Transition Metal Complexes - Attraction
TM complex is highly stable due to attraction between negative ligand charges and the positive metal cation.
Formation of Transition Metal Complexes - Repulsion
Ligand lone pairs act as negative point charges that also repel the negatively charged d-electrons of the metal center, creating local electrostatic repulsion.
Spin State
Number of unpaired d electrons