Bonding, Structure, and The Properties of Matter (exam qus)
1/35
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Describe a method the student could use to compare the reactivity of metal Q with that of zinc. Your method should give valid results.
measure temperature change
when each metal is added to silver nitrate
same concentration/volume of solution
the greater the temperature change, the more reactive
explain why graphite is:
a good electrical conductor
soft and slippert
bonds are covalent
giant structure
three covalent bond per carbon atom so one electron per carbon is delocalised
these delocalised electrons can move through the structure carrying electrical charge so graphite conducts electricity
layered structure of hexagonal rings with weak intermolecular forces between layers
so the layers can slide over each other so graphite is soft and slippery
Explain how a covalent bond holds two atoms together.
electrostatic force of attraction between shared pair of negatively charged electrons and both positively charged nuclei
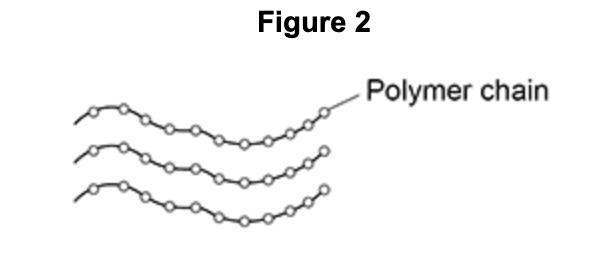
Compare the bonding within the chains with the forces between the chains in this polymer.
covalent bonds between atoms in the chain
intermolecular forces between the chains
covalent bonds are strong
intermolecular forces are weak
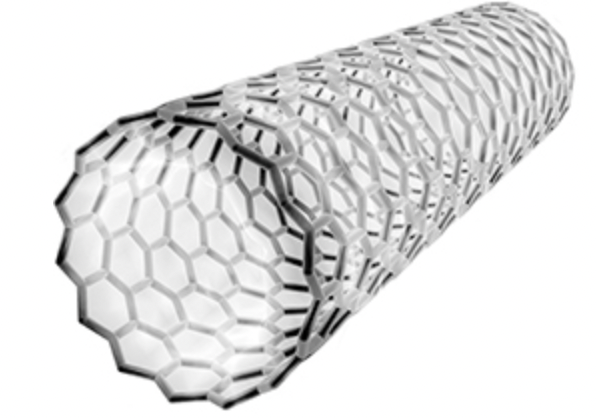
Suggest one property that makes the carbon molecule in Figure 1 useful in nanotechnology.
conducts heat
conducts electricity
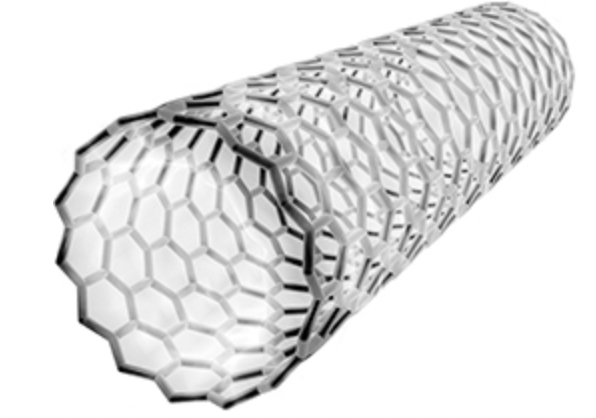
Name the type of carbon molecule in Figure 1.
fullerene
Suggest how anhydrous copper sulfate is used to test for water
Turns white to blue
Describe how copper sulfate solution is obtained from the plants used in phytomining
burned to produce ash
Copper compounds in ash dissolved in sulfuric acid
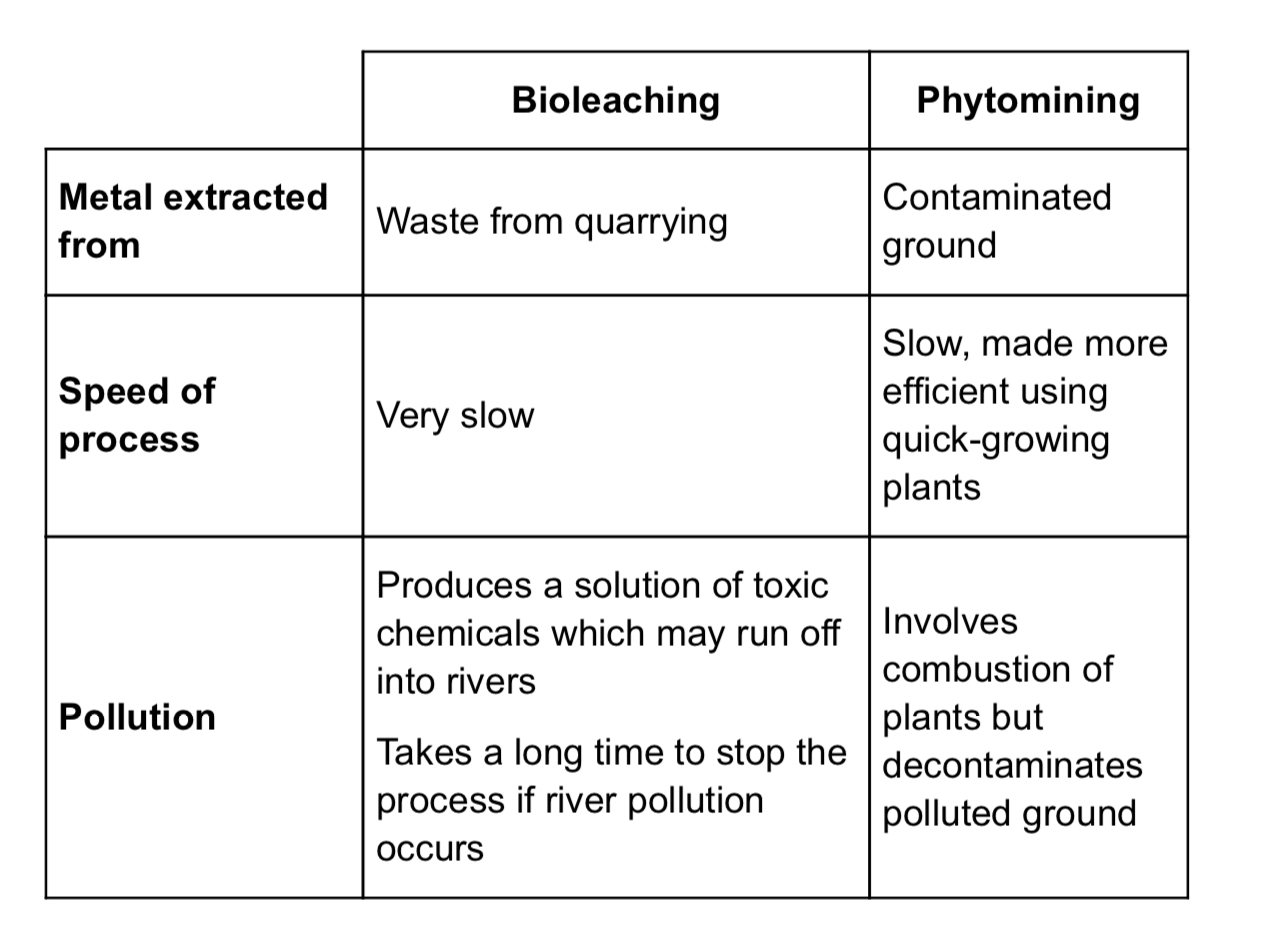
Compare phytomining and bioleaching
Bio leaching is very slow but although slow, phytomining can be made more efficient by growing quick growing plants
Bioleaching extracts copper from quarrying waste but phytomining extracts copper from contaminated ground
Phytomining decontaminates polluted ground but bioleaching can produce toxic run off which may go into rivers
Phytomining takes a long time to stop
Bioleaching is a very slow process
Plants are burned in phytomining
Emissions from car contain co2.
Explain why co2 emissions during the use and operation are not the total carbon footprint for a car.
Refer to LCA
extracting raw materials
Disposal at the end of life
Manufacturing
Compare the bonding within the chains with the forces between the chains in this polymer
bonding within the chains are covalent and are strong
Forces between the chains are intermolecular and weak
Suggest one property that makes the carbon molecule in figure 1 useful in nanotechnology
conducts heat
Conducts electricity
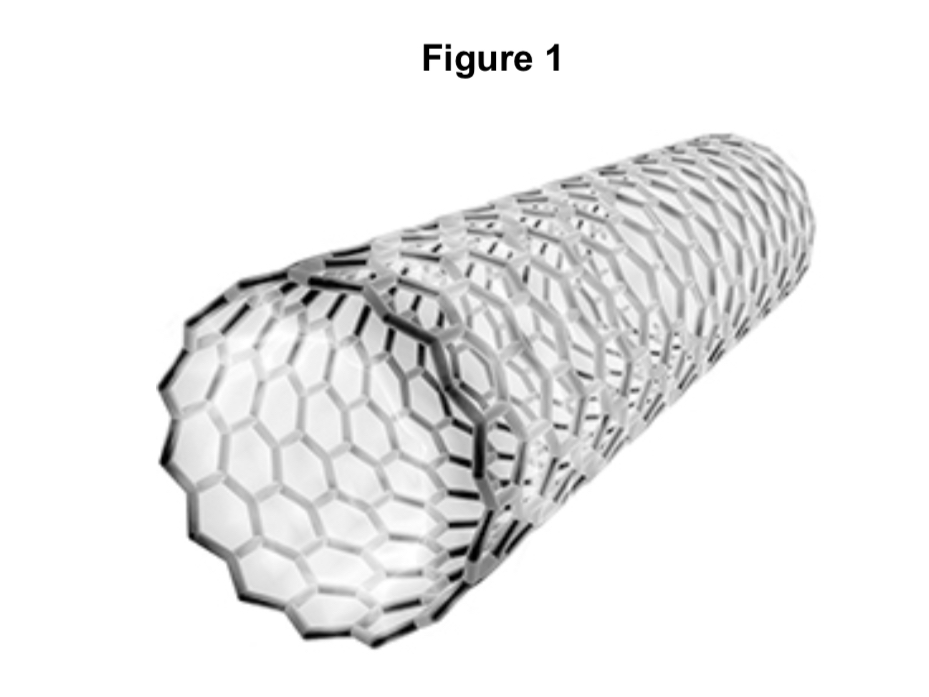
Name the type of carbon molecule in figure 1
Fullerene
* a good electrical conductor
* soft and slippery
You should answer in terms of structure and bonding.
* giant structure
\
a good electrical conductor
* only 3 electrons per carbon atom used in bonds so one is delocalised
* these delocalised electrons can move through the structure carrying electrical charge
* so graphite conducts electricity
\
soft and slippery
* layered structure of hexagonal rings with weak intermolecular forces between layers
* there are no bonds between the layers so the layers can slide over each other
* so graphite is soft and slippery
* outer shell is further from the nucleus
* there is less attraction between the nucleus and the outer shell
* the atom is able to lose an electron more easily
* with strong electrostatic forces of attraction between ions
* therefore large amounts of energy are needed to break the bonds
* carbon dioxide
* magnesium oxide
* silicon dioxide.
* carbon dioxide is small molecules with weak intermolecular forces
\
* all 3 compounds have strong bonds
* co2 and sio2 are formed from 2 non metals and therefore are covalent
* so electrons are shared
* mgo is formed with a metal and a nonmetal so bonds in mgo are ionic
* so electrons are transferred from mg to o (2 electrons transferred)
\
* bonds in sio2 are single bonds where each silicon forms 4 bonds and each oxygen form 2 bonds
* in co2 the bonds are double bonds where carbon forms 2 double bonds and oxygen forms one double bond
Answer in terms of nitrogen’s structure.
Suggest why it is cheaper to use nanoparticles of silver rather than coarse particles of silver.
Other substances are added to silicon dioxide to make glass. Glass melts at a lower temperature than silicon dioxide.
Suggest why.
* iodine gains 1 electron
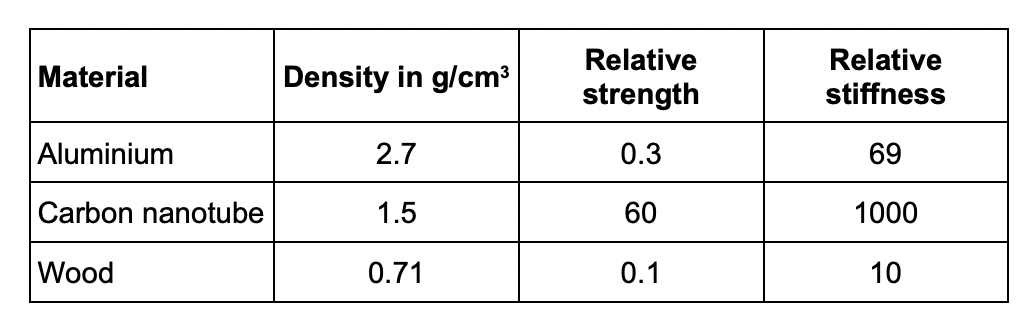
* aluminium is the most dense so it will make the racket heavy
* carbon nanotube is the strongest so less likely to break
* wood and aluminium are too weak so they will break more easily
* carbon nanotube is the stiffest so least likely to bend out of shape
* wood and aluminium are not very stiff so could bend out of shape
\
conclusion
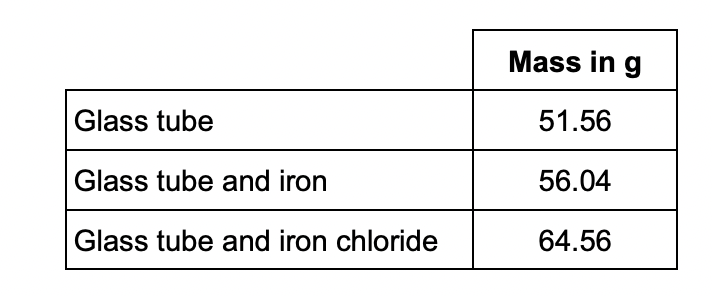
moles of iron atoms : moles of chlorine atoms
Determine the balanced equation for the reaction.
Relative atomic masses (Ar): Cl = 35.5 Fe = 56
* iron moles = 4.48/56 = 0.08
* chlorine moles = 8.52/35.5 = 0.24
* 0.08:0.24 = 1:3
\
2Fe + 3Cl2 → 2FeCl3
Both metals are more reactive than silver.
The student is provided with:
• silver nitrate solution
• metal Q powder
• zinc powder
• a thermometer
• normal laboratory equipment.
No other chemicals are available.
Describe a method the student could use to compare the reactivity of metal Q with that of zinc.
Your method should give valid results.
* make sure there is the same concentration of solution and mass of the metal
* the greater the temperature change, the more reactive the metal is
In your method you should name the apparatus you will use.
You do not need to mention safety.
* filter excess mg using filter paper and funnel
* pour solution into evaporating basin
* heat using bunsen burner
* leave to crystallise and pat dry afterwards
Use the information in the figure above to help you.
In your method you should name all of the apparatus you will use.
* add until copper carbonate is in excess
* filter the excess carbon carbonate using filter paper and funnel
* pour solution into evaporating dish
* heat using bunsen burner
* leave to crystallise and pat dry
* wear safety goggles
Use the above statement to predict and explain how the overall energy change for the reaction of ethene with chlorine will differ from the overall energy change for the reaction of ethene with bromine.
* Cl-Cl and C-Cl bonds are stronger then C-Br bonds and Br-Br bonds
\
* more energy is required to break bonds with chlorine
* more energy is given out when making bonds with chlorine
\
* if Cl-Cl bond changes more, then less exothermic
* if C-Cl bond changes more then more exothermic
* can’t tell how overall energy change will differ as we do not know which changes more
Why does manganese oxide conduct electricity as a liquid?
Ions move around in the liquid
Describe what happens when a lithium atom reacts with a chlorine atom. Answer in terms of electrons.
lithium loses one electron
chlorine gains one electron
transfer of one electron to form positive and negative ions
The relative formula mass (Mr), in grams, of sodium fluoride is one _______________ of the substance.
mole
one advantage of using nanoparticles in sun creams
better coverage
disadvantage of using nanoparticles in sun creams
potential cell damage