Clinical Biochem week 2 flashcards
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Photometry
“light measurement”
any method measuring light intensity
classified by the manner light is measured
Compares an unknown [sample] to a known [standard]
absorbance/transmittance
[SAMPLE] determined by measuring amount of light absorbed/transmitted
compared to the amt of light absorbed/transmitted by STANDARD solution
Colorimetry
when the chemical reaction occurs, there is a color change
Emission
luminescence, chemiluminescence
sample absorbs energy (light, heat) → emits light
[SAMPLE] directly proportional to light emission
applications: luminescence methods
sample absorbs energy
Scatter
particle in solution scatter light
[SAMPLE] proportional to light scattered
Applications: turbidimetry; Nephelometry
Reflectance
light strikes a sample on a reflective surface
[SAMPLE] inversely proportional to light reflected
higher [ ], less light reflected
applications: reflectance methods
Wavelength
distance between 2 peaks in nm = 10^-9nm
amplitude
light wave height → light intensity
Light dispersion (spectrum)
white light bends and wavelength’s separate
different refractive indices
longer wavelength ROYGBiV — shorter wavelength
visible wavelength = 380nm - 760nm
Photometers
measure light intensity absorbed / transmitted by a sample solution
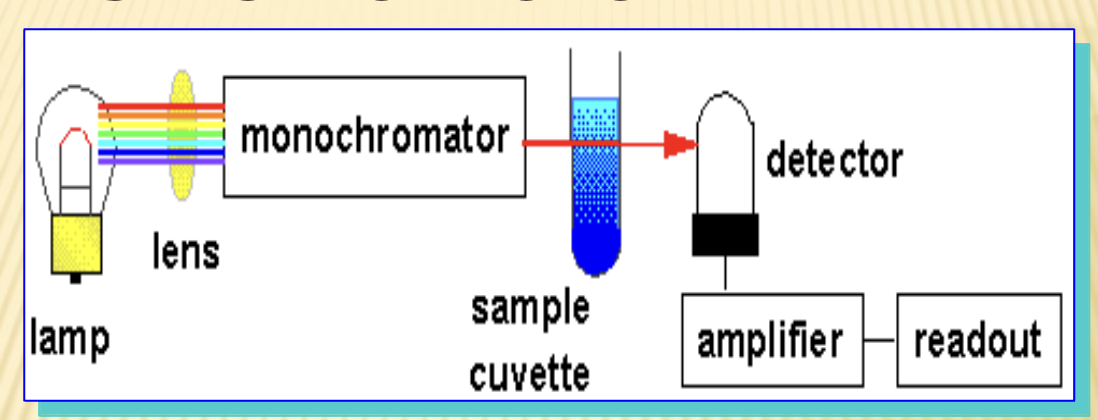
Spectrophotometer
measures monochromatic light absorbed/transmitted by a solution
Light source: tungsten, hydrogen/Deuterium
monochromator: filters, diffraction gratings
Cuvet: Quartz
Transmittance (%T)
monochromatic light strikes a solution
some light absorbed, remainder transmitted through a solution
%T = light transmitted
light striking sample x 100
%T v. [ ] is non-linear relationship
![<ul><li><p>monochromatic light strikes a solution</p></li><li><p>some light absorbed, remainder transmitted through a solution</p><ul><li><p>%T = <u>light transmitted</u></p><ul><li><p>light striking sample x 100</p></li></ul></li></ul></li><li><p>%T v. [ ] is <u>non-linear</u> relationship </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/bb916f28-9c2e-47d8-b9ea-66ef5005507e.png)
Absorbance (A)
measure of monochromatic light absorbed by sample
molecules absorb light at specific wavelengths
monochromatic light ensures accuracy
A is directly proportional to concentration
not measured directly by a photometer
A= 2-log%T
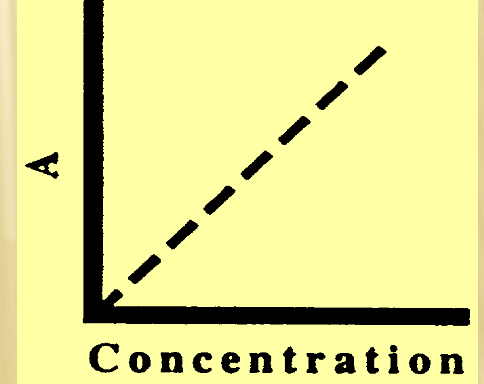
Beer’s Law
A directly proportional to [ ]
Lambert’s Law
A = # of light absorbing molecules in lightpath
Beer-Lambert Law
A= abc
A absorbance
a = molar absorptivity
b = lightpath (cm)
c = concentration

Using Beer’s Law for results
standard curve
absorbance vs. concentration
construct curve using “best fit” line from >1 STANDARD
must go through origin
upper linearity to [Highest STANDARD]
Calculation
1 standard
upper linearity must be established
Analytical sampels
reagent blank
corrects light absorbed by reagent from final sample absorbance reading
standards (calibrator)
sample
Light source
routine
visible and near UV (340nm) and IR wavelength
tungsten, quartz-halogen
special
strict UV
hydrogen, deuterium
pure monochromatic light
lasers
monochromator
produces monochromatic light (1 color)
Bandpass (Bandwidth)
amount of light directed at sample
wavelength range produced
550 nm selected 525-575 nm light produced
bandwidth = 50 nm
describes light purity
narrow = more pure
wide = less pure
Monochromator types
Filters (wider bandwidth)
transmit wanted λ and absorb unwanted λs
Diffraction gratings (narrow)
mirror-like material with etched parallel grooves
Source light strikes the grating → each groove produces a linear spectrum
more grooves → smaller bandwidth
Sample cuvet/cells
material
UV work → quartz glass or special plastic
pathlength = 1 cm
cuvet chamber shielded
stray light
light reaching detector at a λ other than selected
all light not passing through sample
causes false decrease in absorbance
Photodetector
detects light, converts to electrical signal and sends to a readout device
Photomultiplier tube (PMT)
detects and amplifies initial signal up to a million times
photodiodes
silicon chips convert light to electrical signal
can measure many λs simultaneously
Reflectance photometry
Reflectometry
monochromatic light directed at a flat surface at a 45° angle
reagents impregnated on flat test surface → reacted with sample
indirectly proportional
Detector
positioned at 90° angle to test surface
Chromophores
some light gets absorbed by chromophores on test surface
unabsorbed light reflected
measured by detector as “reflection density”
Reflection density indirectly proportional to [ ] and non-linear
Algorithim linearizes (correction factor makes it linear)
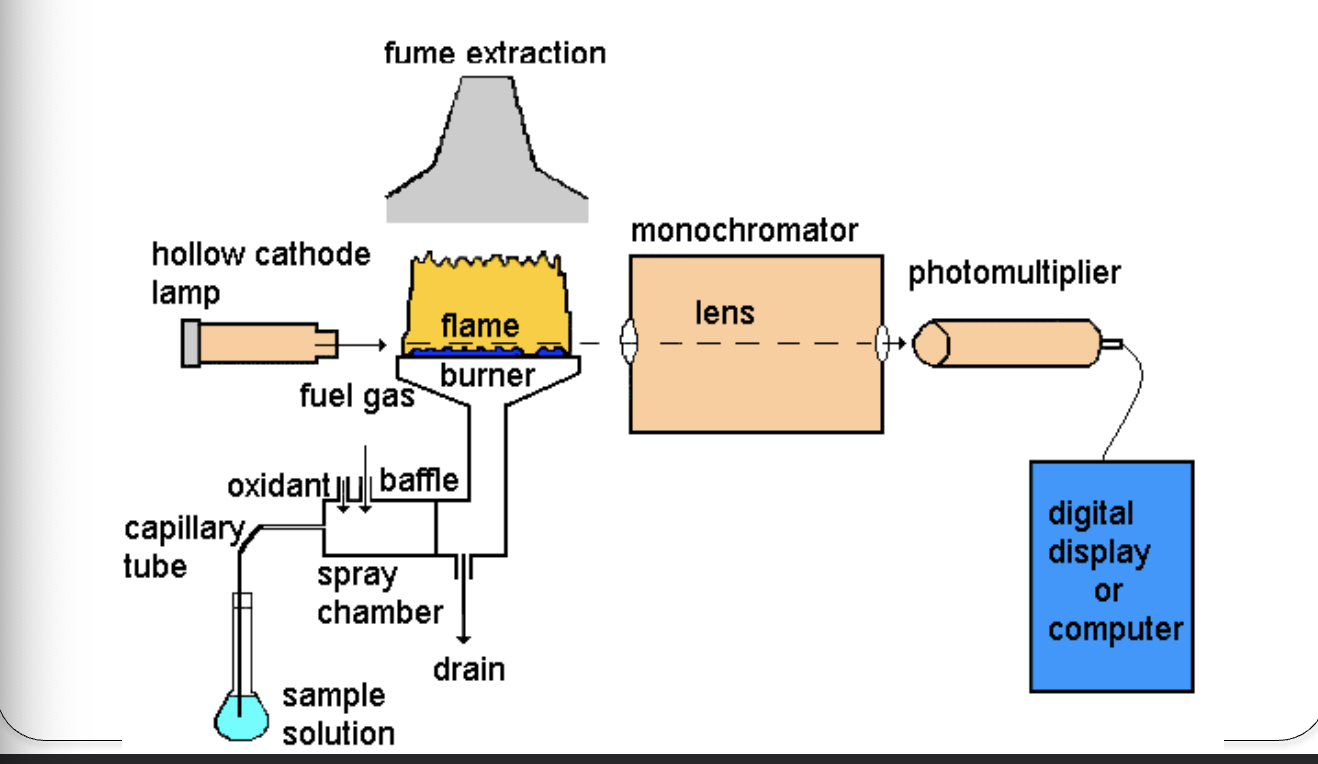
Atomic absorption spectrophotometry
Analytes
calcium, magnesium, trace metals
sensitive and specific
Principle
Ground state atoms (sample) in the flame absorb specific light λ
[ ] directly proportional to absorbance
AAS
analogous to absorption spectrophotometry (atoms vs. molecules)
Hollow cathode lamp → analyte-specific light source
flame: sample holder
Hollow cathode lamp
specific light λ
passes through flame containing sample atoms
ground-state atoms specifically absorb λ
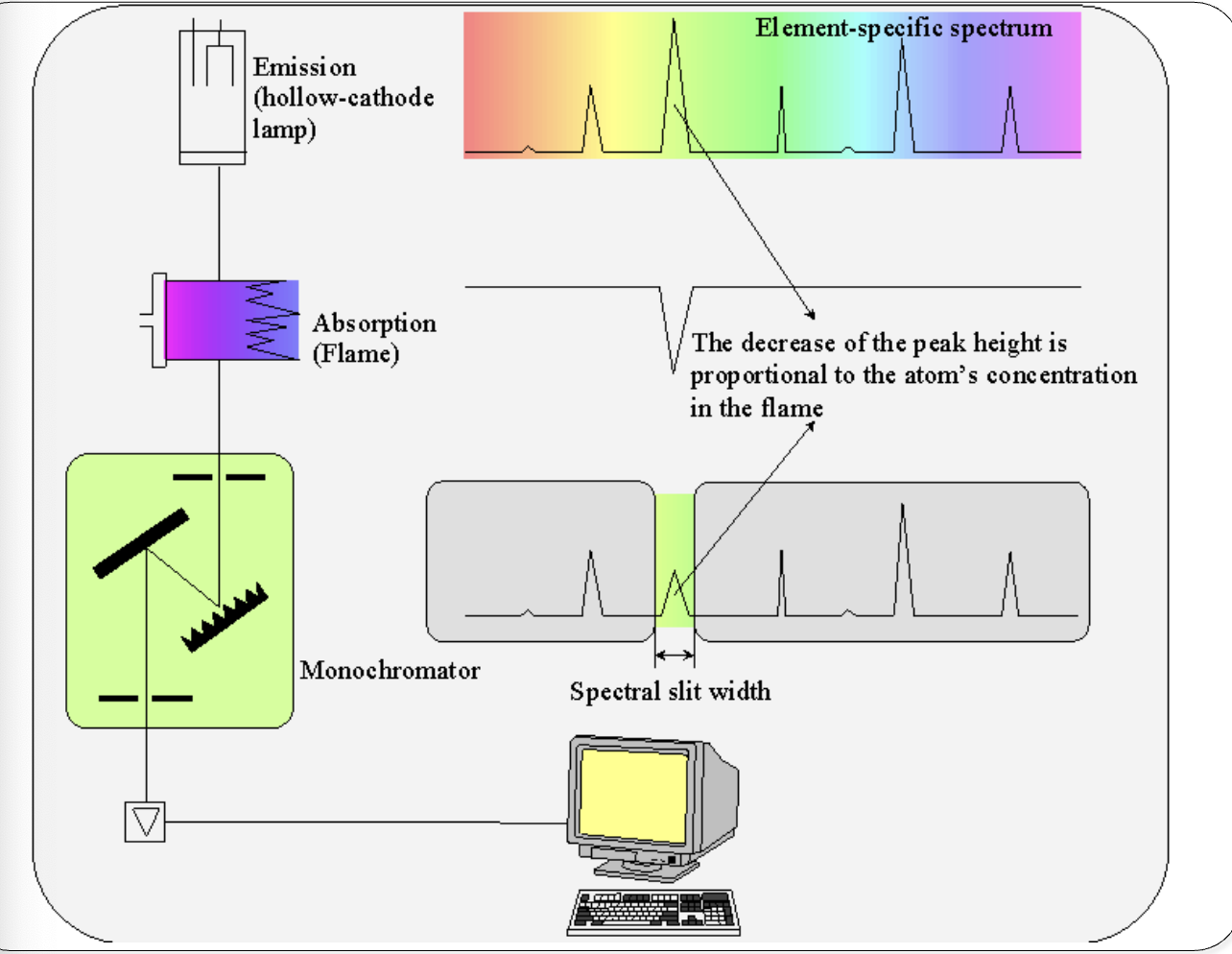
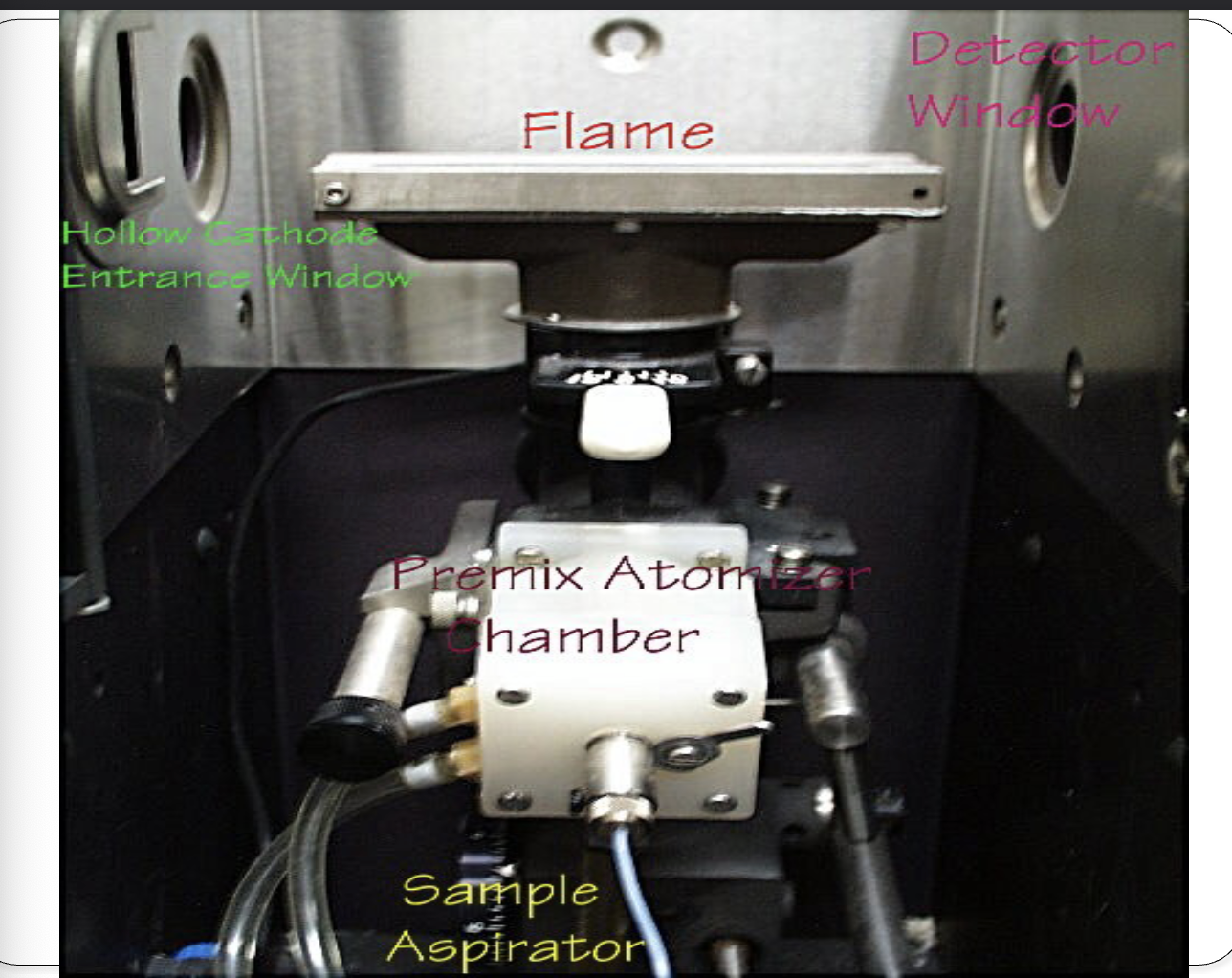
Sample handling
sample chamber
atomizer
vaporizes sample, mixes with fuel/oxidant
Acetylene/air
flame
reduces atoms to ground state
Flameless AAS graphite furnace
sample
carbon rod well or metal strips
heat sample to atomize
Principle (same as Flame AAS)
hollow cathode light source passed through atomized sample
ground state atoms absorb specific light λ