IB Biology - Molecular Biology
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
Name the most frequent elements in life
CHON (Carbon, hydrogen, oxygen, nitrogen, and are main elements found in organic molecules in organisms)
5 other necessary elements for life
Sulfur: found in 2 amino acids
Calcium: chemical messenger that helps regulate cell processes
Phosphorus: ATP and DNA, RNA (phosphate)
Iron: needed in cytochromes for electron transport chains, hemoglobin
sodium: water balance/homeostasis, pumped into cells for water uptake, nerves
Compound
2 or more elements combined in a fixed ratio
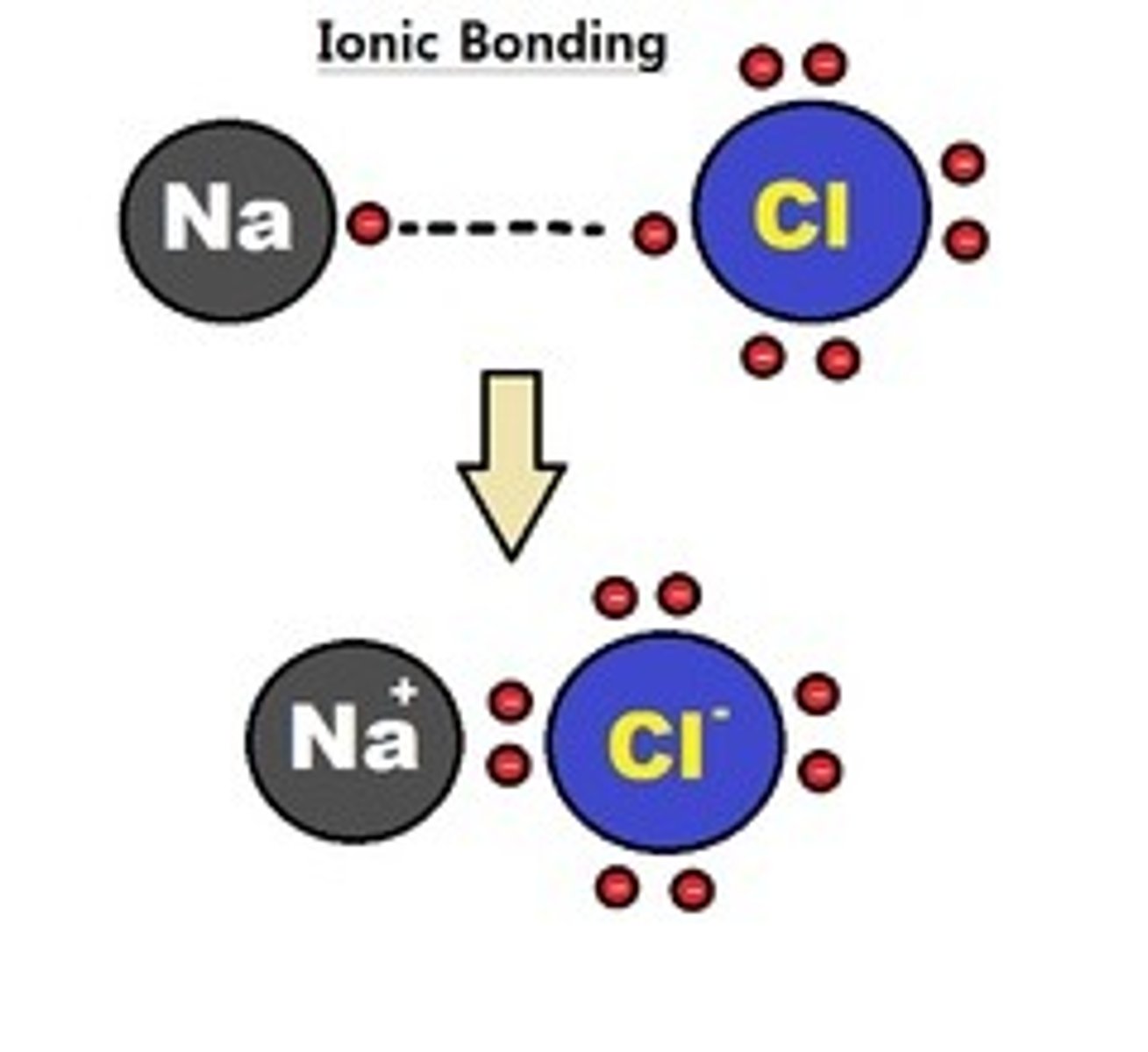
Ionic bonds
one atom strips valence electrons from another atom (high electronegativity difference), electron transfer creates ions (charged atoms)
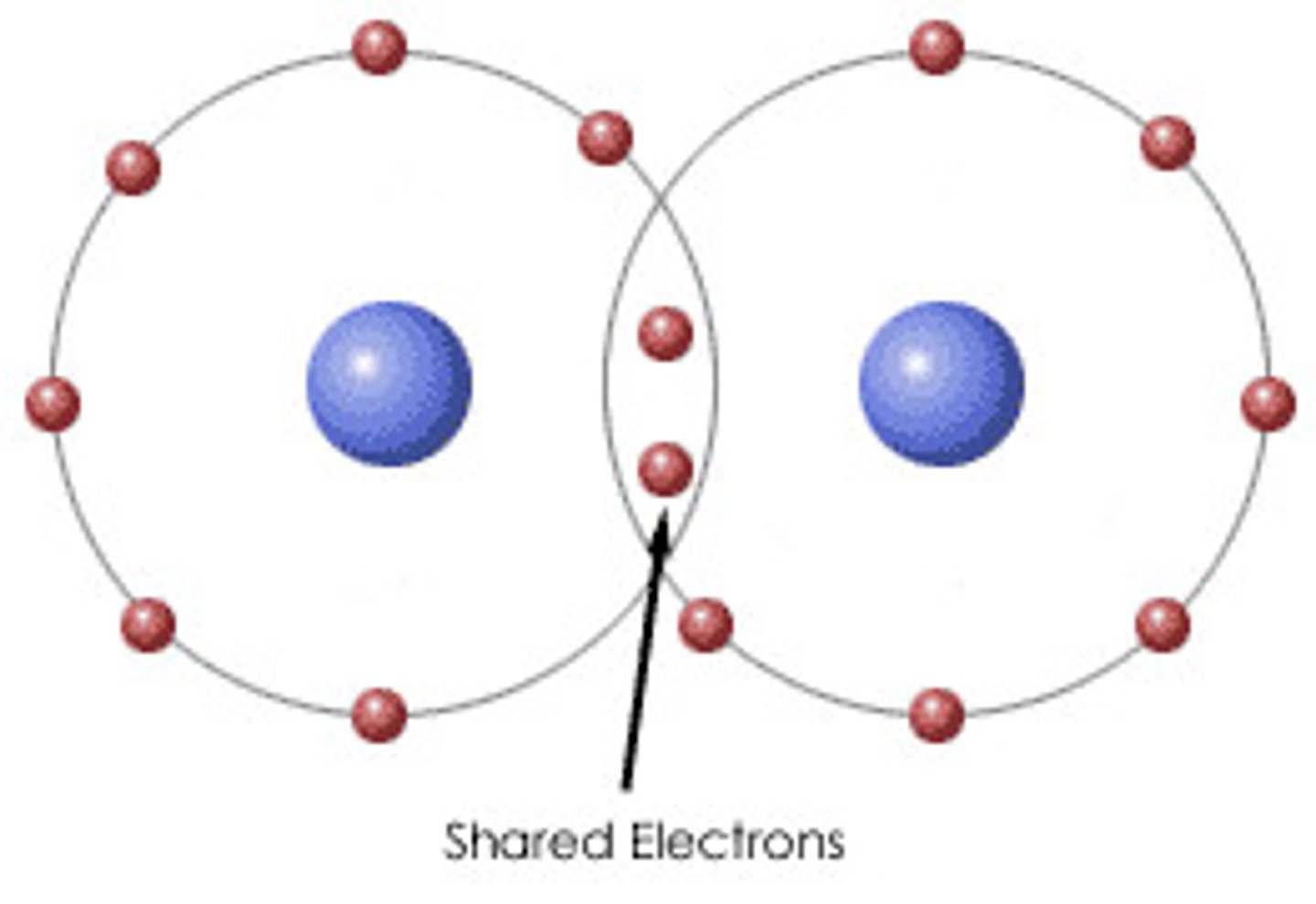
Covalent bonds
sharing pair of valence electrons, # of electrons required to complete an atom's valence shell determines how many bonds will form
Molecule
elements covalently bonded
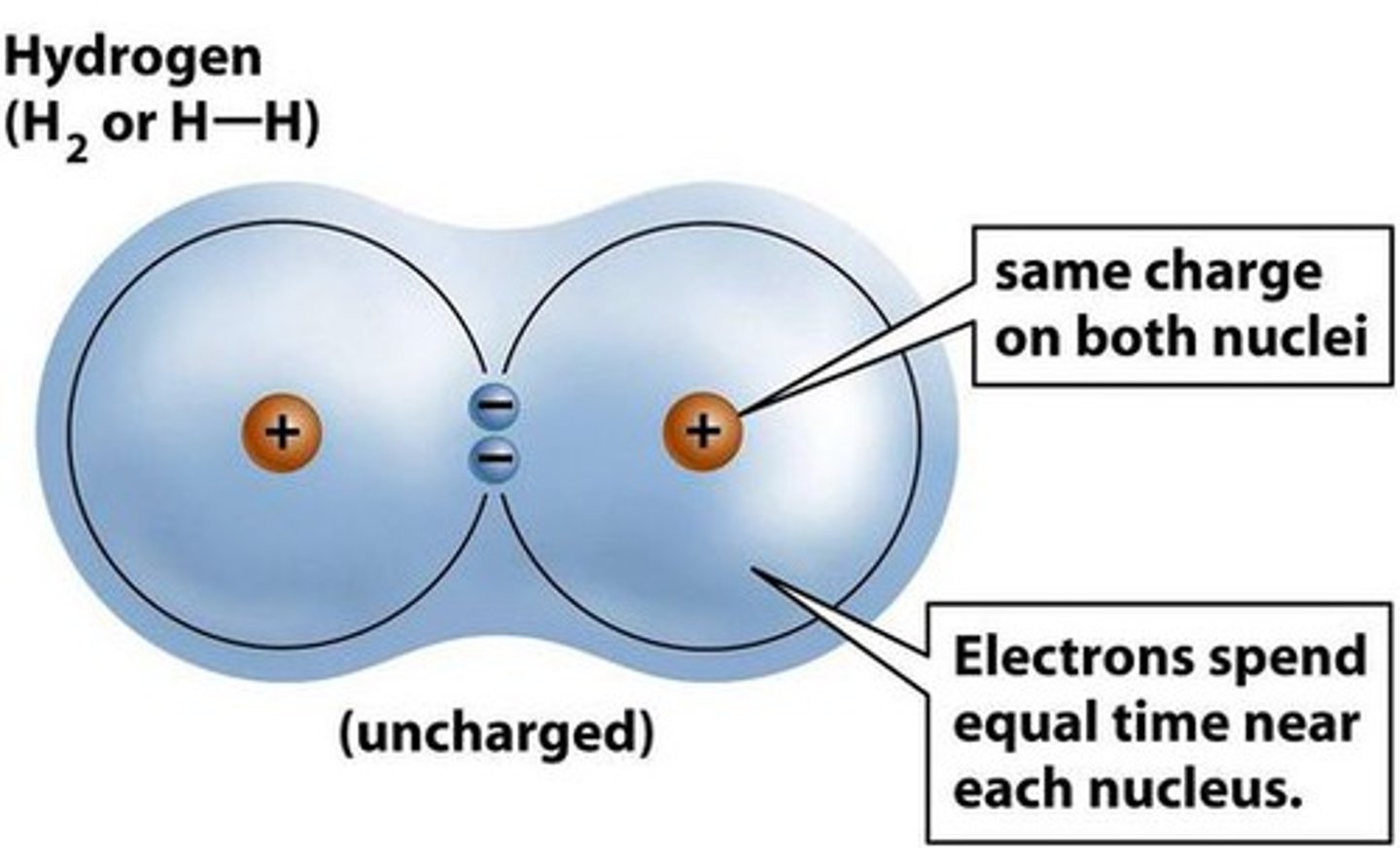
Nonpolar covalent bonds
Electrons shared equally
Polar covalent bonds
One atom more electronegative than the other (creates partial charges)
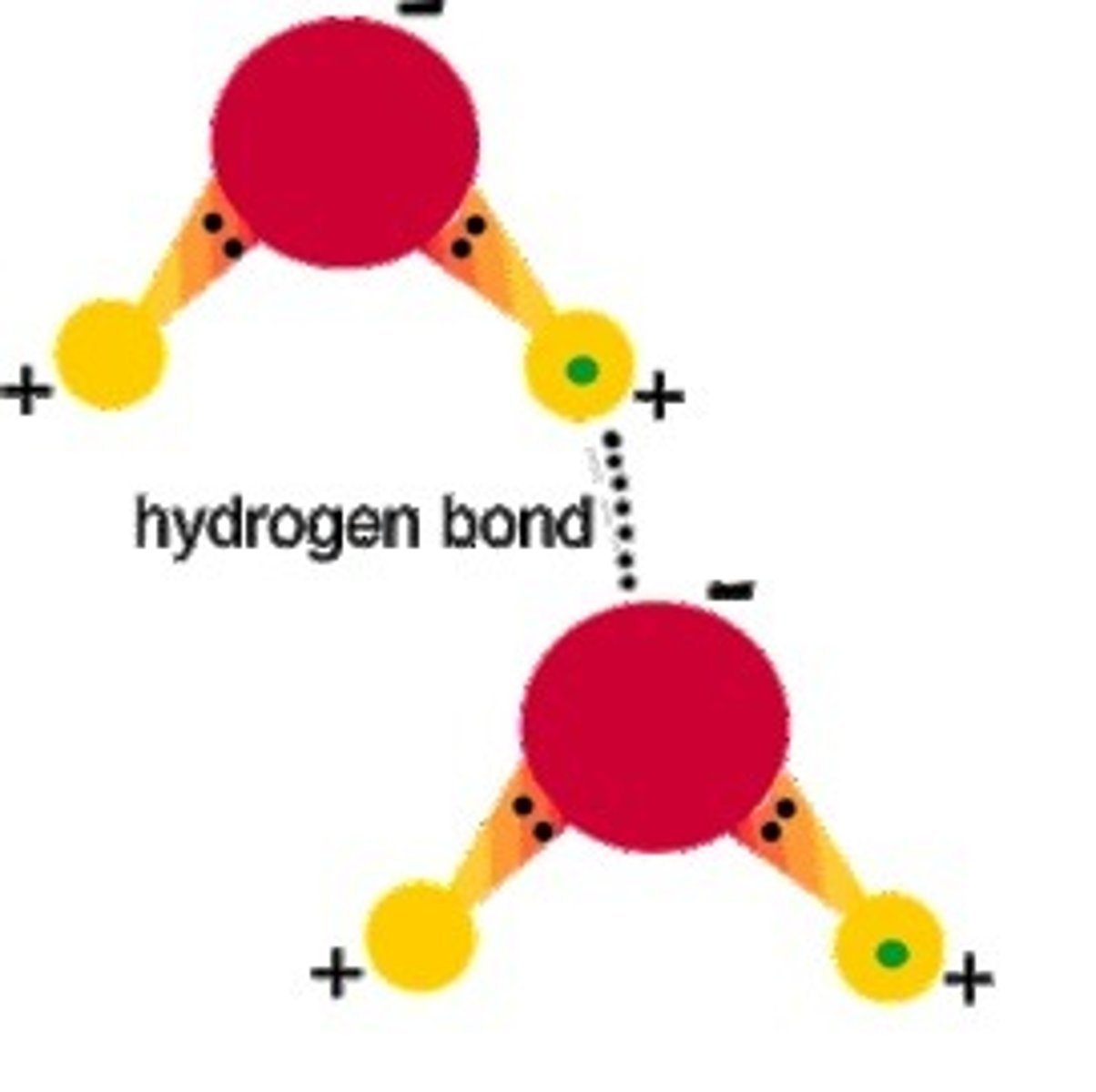
Hydrogen bond
Attraction (weak) between a partially positive H atom of one polar molecule and a partially negative atom (O or N) of another polar molecule
What 2 things can the many properties of water be attributed to?
Its polarity and hydrogen bonds
List the thermal properties of water
High specific heat, high heat of vaporization, and high boiling point
High specific heat
(Amount of heat absorbed)/(amount of heat lost/change temperature by 1°C)
(1 cal)/(g/°C)
High heat of vaporization
quantity of heat required to convert 1g from liquid to gas states (Evaporative cooling/sweat)
High boiling point
Water has a boiling point of 100°C

Compare the properties of water vs. methane (think thermal properties)
Water has almost 2x the specific heat capacity of methane, water heat of vaporization is almost 3x that of methane, melting and boiling points of methane are lower than water
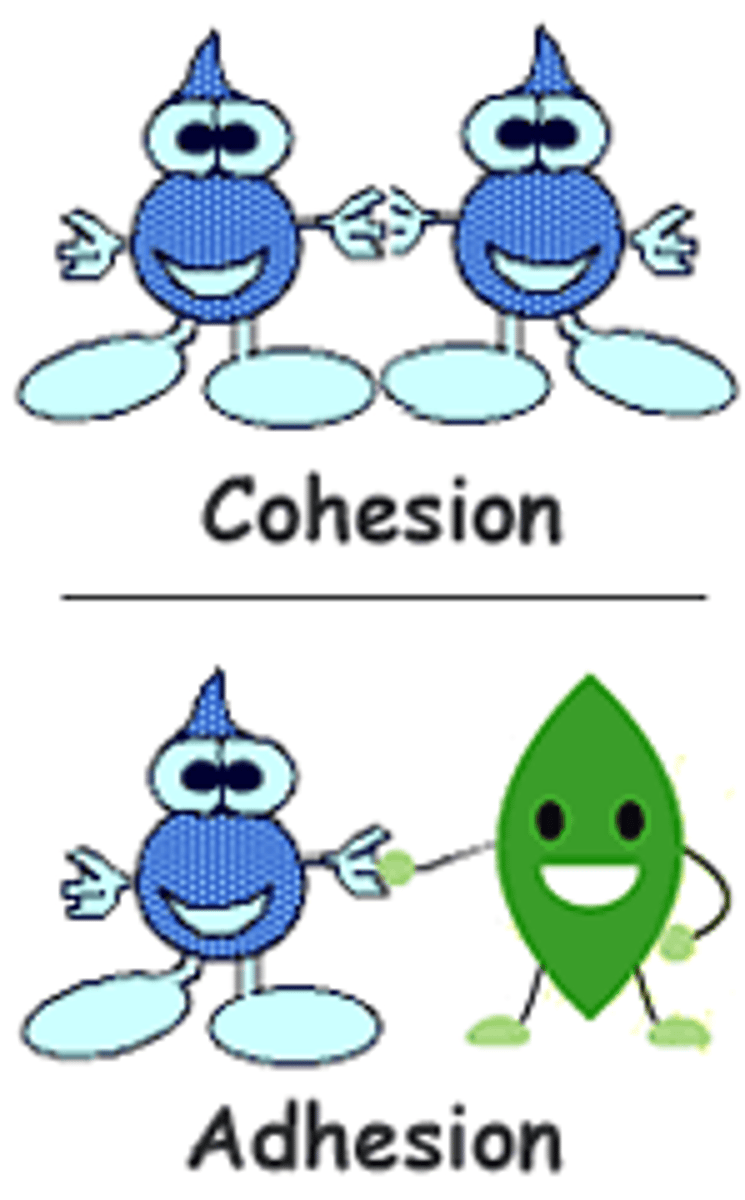
List the cohesive properties of water
Cohesion, surface tension
Cohesion
H bonds holding water molecules together
Surface tension
Measurement of the difficulty to break or stretch the surface of a liquid

List the solvent properties of water
-water is a very good solvent, sometimes called the "universal solvent"
-substances that are able to dissolve in water are polar or ionic
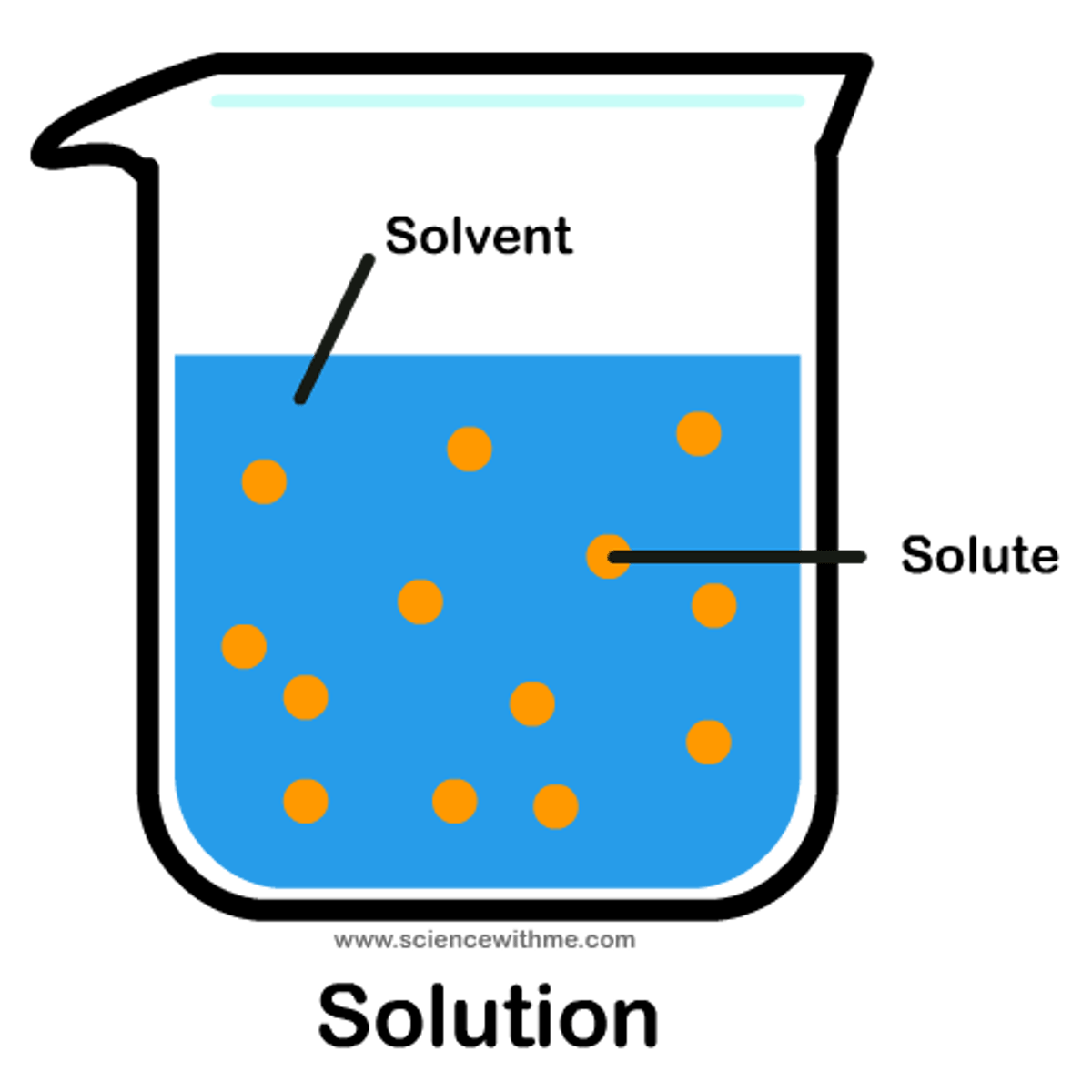
Solution
completely homogenous mixture, diffused solute in solvent
What does transport in blood depend on?
Solubility
What are some substances that are transported in blood?
-NaCl: dissolved in plasma (ions)
-amino acids: dissolved in plasma (sufficient solubility due to charged regions)
-glucose: dissolved in plasma (polar)
-oxygen gas: carried by hemoglobin (nonpolar)
-fats: transported in lipoprotein complexes (nonpolar)
-cholesterol: transported in lipoprotein complexes (nonpolar) HDL and LDL
Adhesion
H bonds holding molecules to another substance
Density property of water
Water is less dense as a solid than as a liquid, due to H bonding. Crystalline lattice keeps molecules at a distance.
Vitalism
The belief that living organisms possess a non physical inner force or energy that gives them the property of life
Falsification of Vitalism
When urea (organic compound in liver) was artificially synthesized by German chemist Friedrich Wöhlerfor the first time, vitalism was deemed false.
Organic
Carbon compounds found in living organisms, almost all carbon compounds are organic
Inorganic
Not consisting of or deriving from living matter.

Why is carbon important?
Tetravalent (can form 4 covalent bonds), which allows it to produce a variety of stable organic compounds
Metabolism
web of all enzyme-catalyzed reactions in a cell or organism
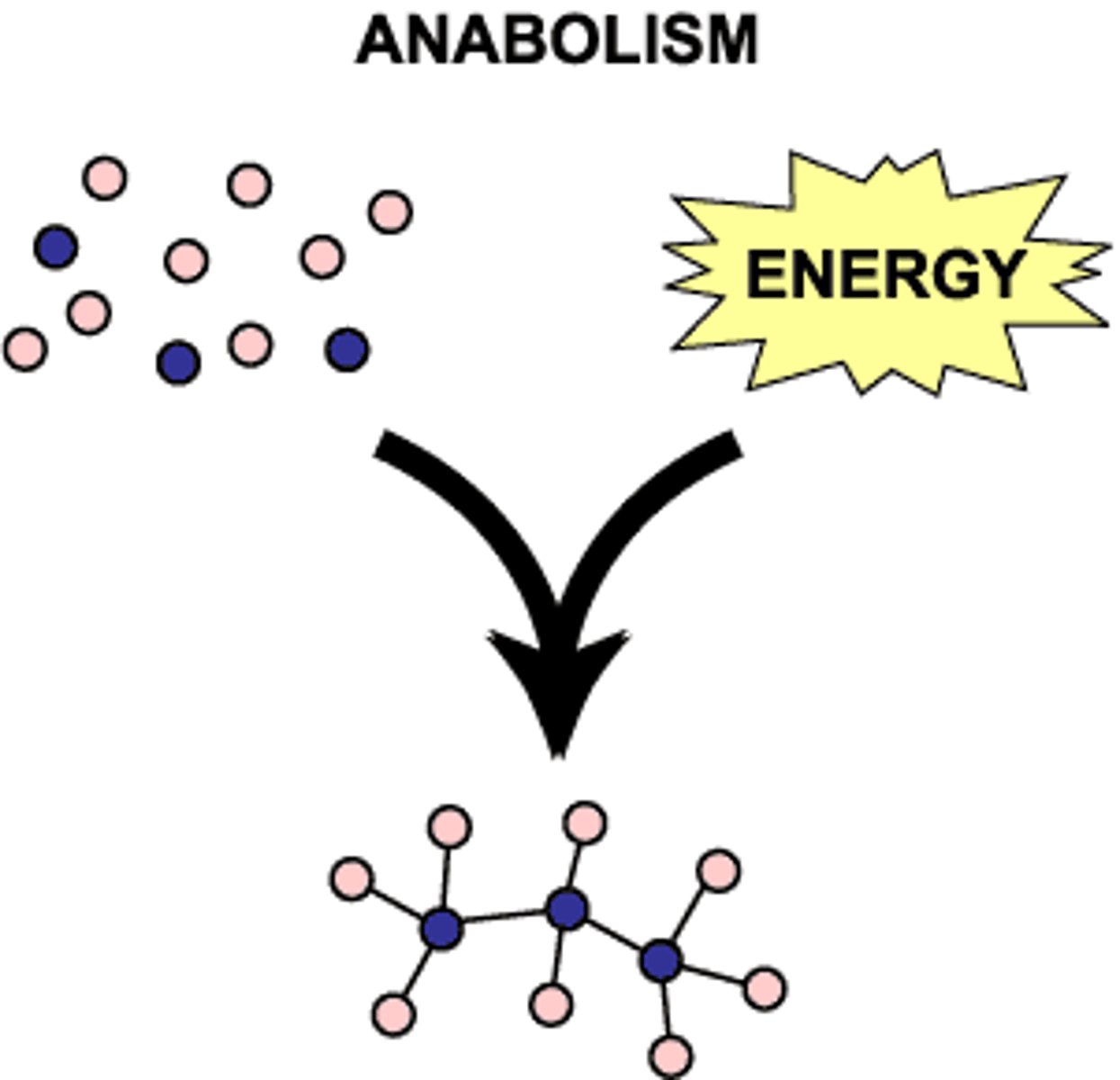
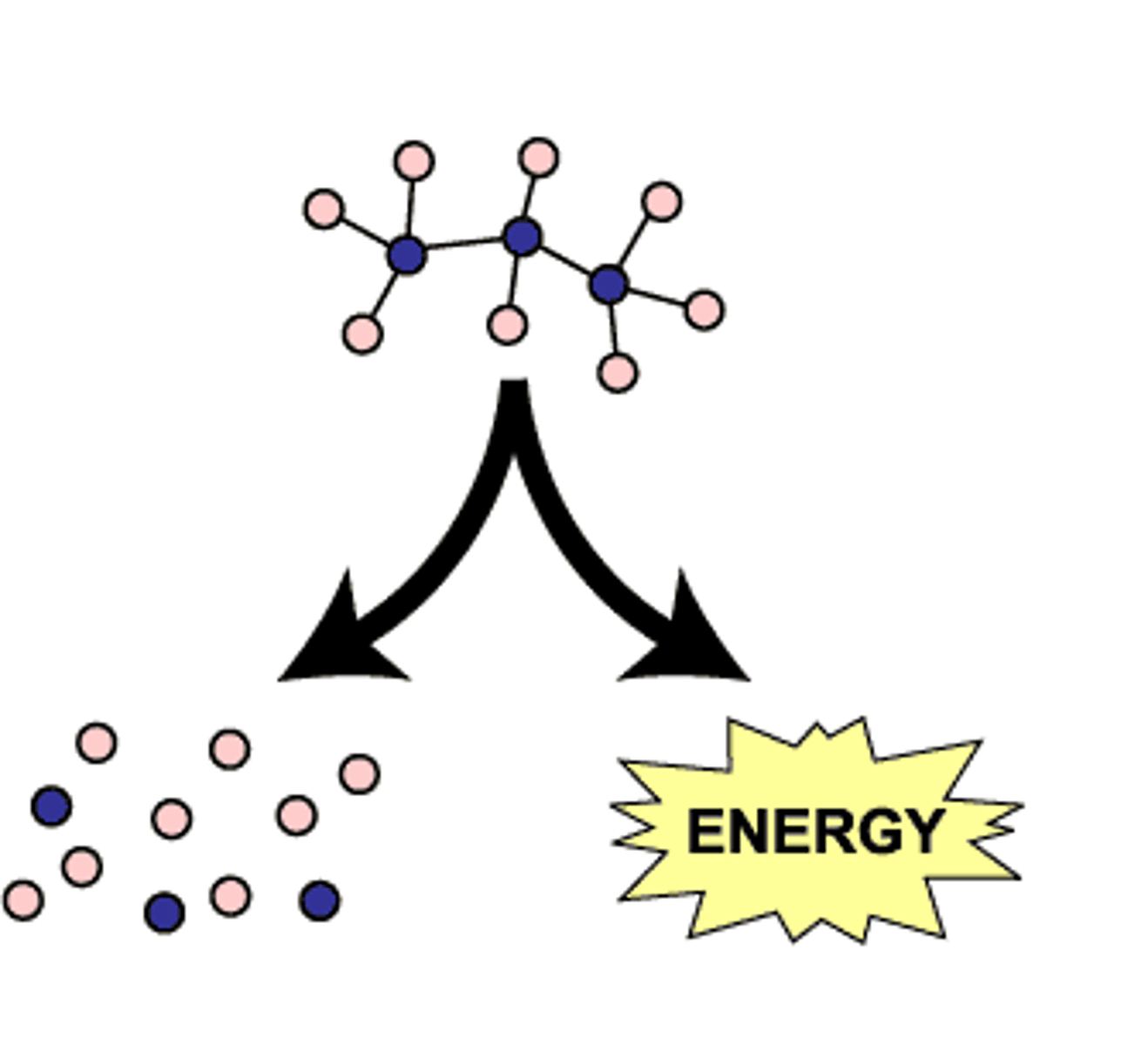
Anabolism
synthesis of complex molecules from simpler molecules (ex. making DNA, photosynthesis)
Catabolism
breakdown of complex molecules into simpler molecules (ex. digestion, respiration)
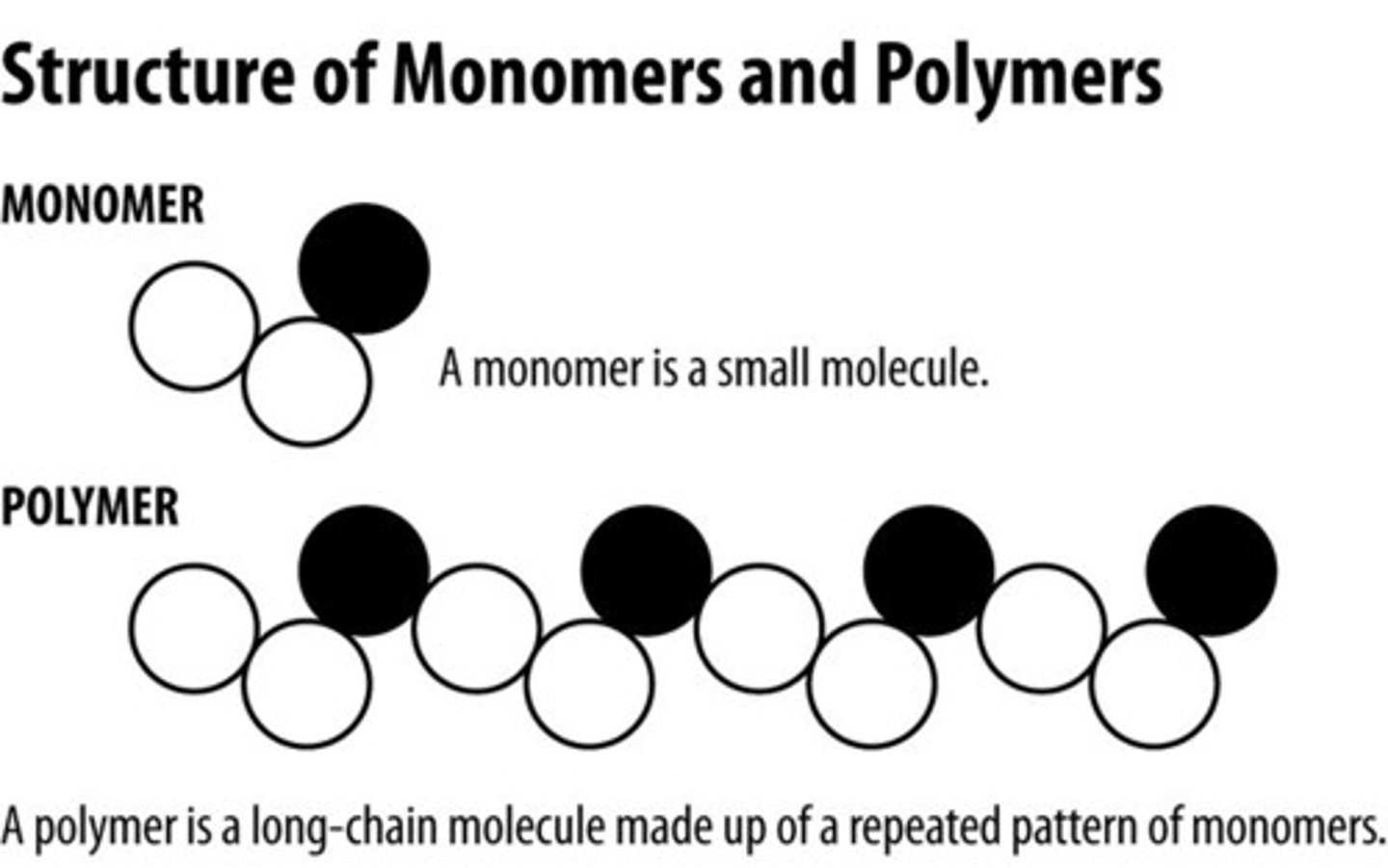
Polymers
3 or more covalently bonded monomers (subunits)
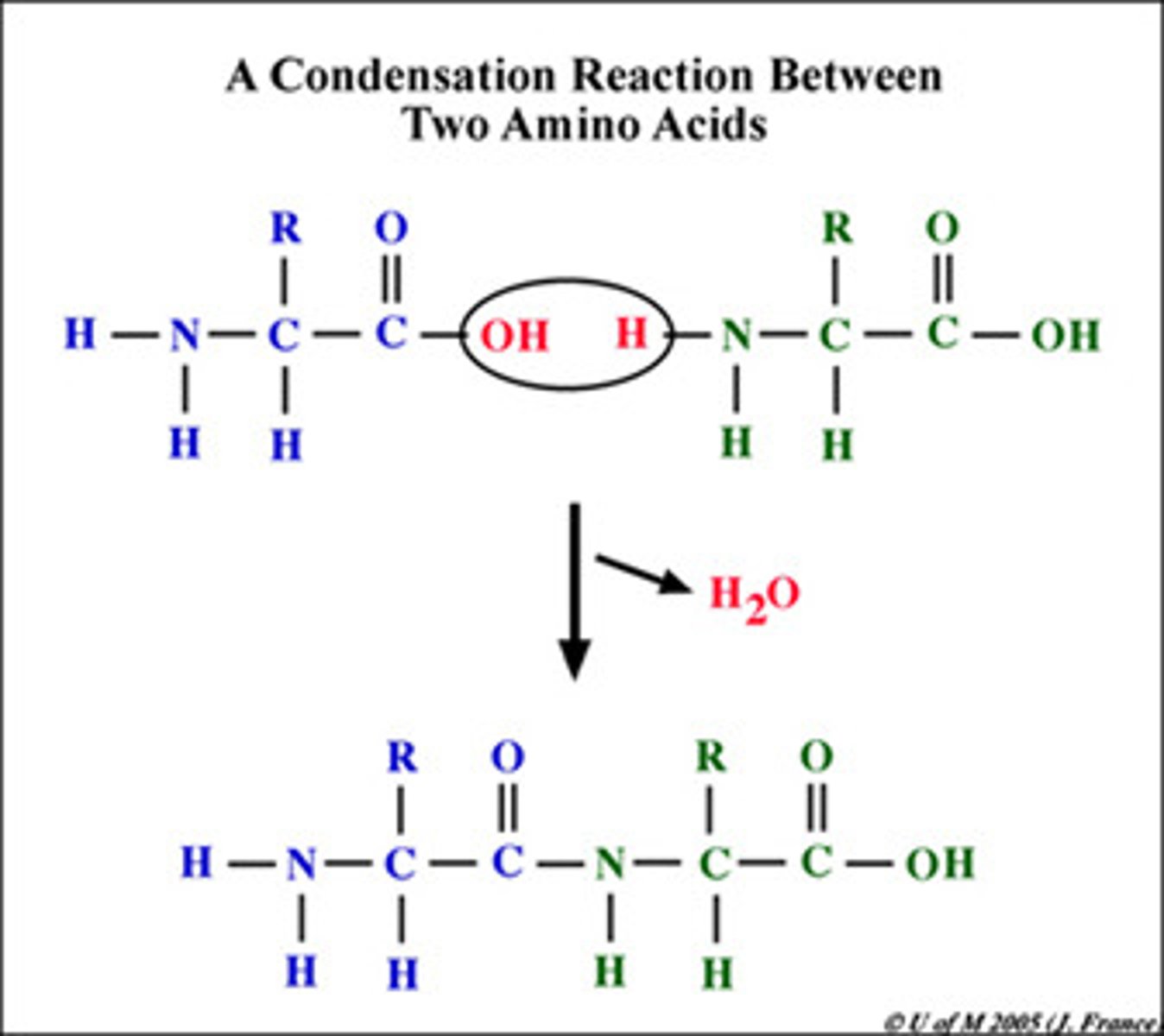
Condensation reaction/dehydration synthesis
Joins monomers: one monomer provides a hydroxyl group while the other provides a hydrogen to form a water molecule, which is removed.
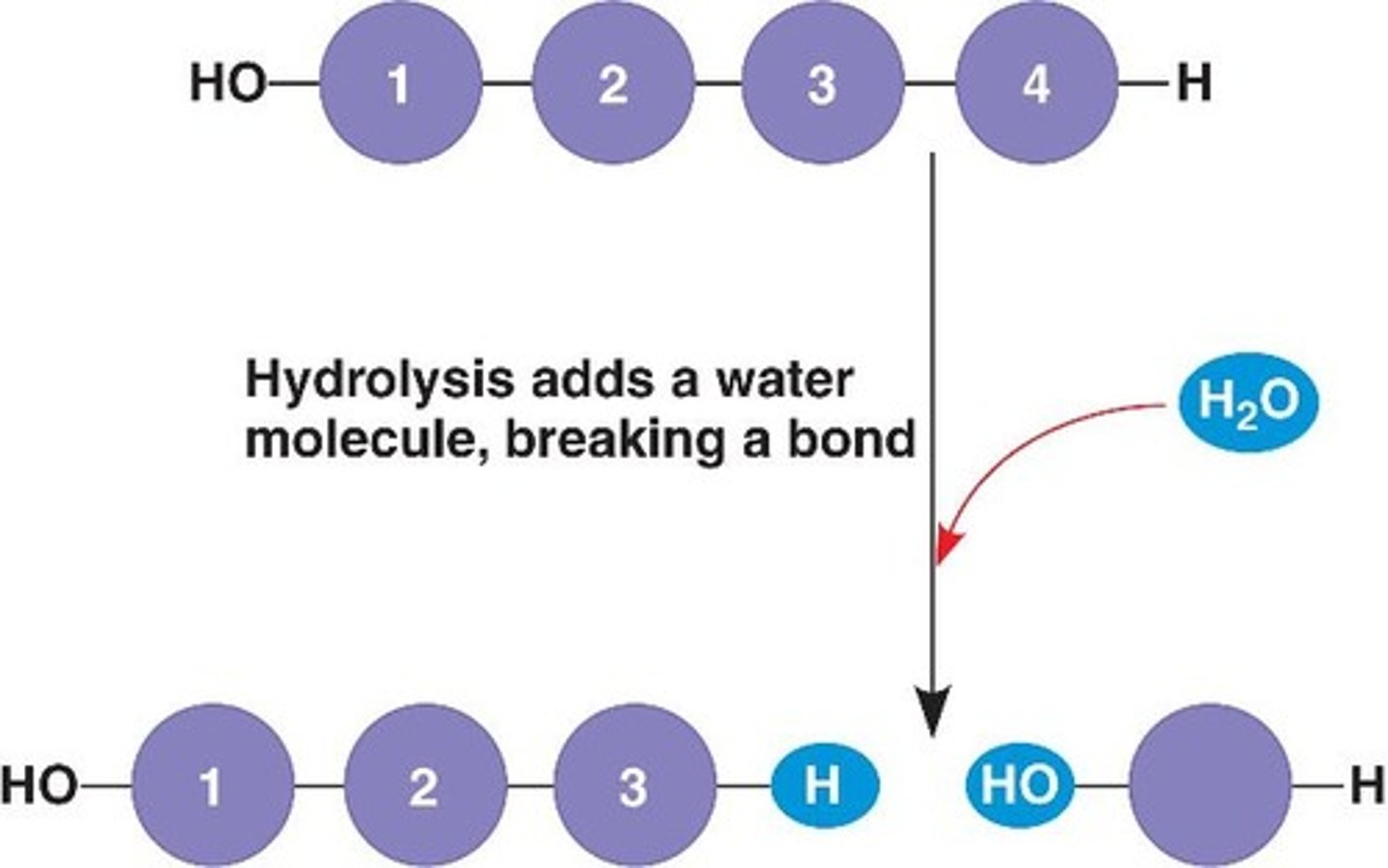
Hydrolisis
bonds between monomers are broken by adding water (digestion)
Monosaccharides
CH2O=empirical formula
Examples:
glucose- blood sugar
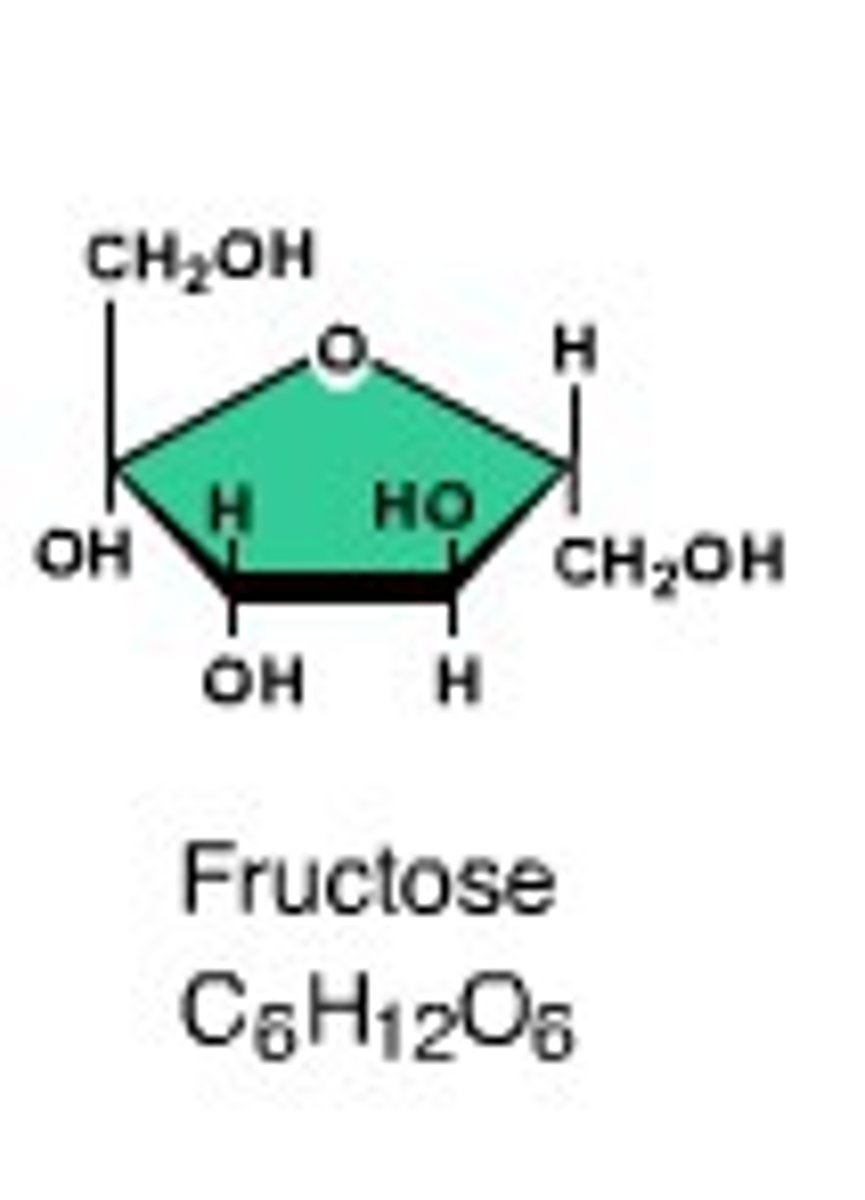
fructose- fruit sugar
galactose
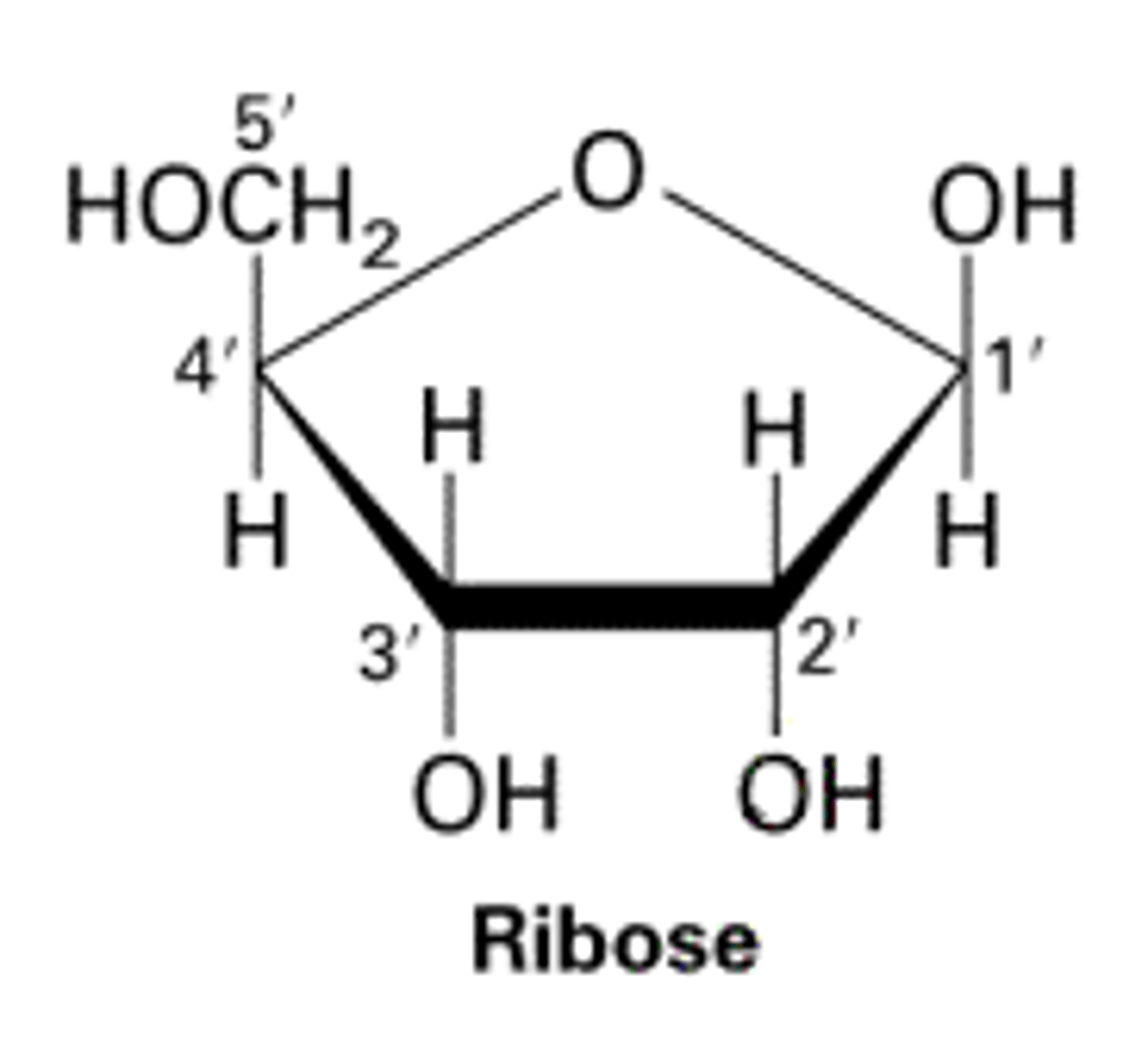
ribose- structural component in RNA
Importance:
-produced by photosynthesis
-used in cellular respiration (to make energy)
-summary: energy storage and release
-also used to build structures
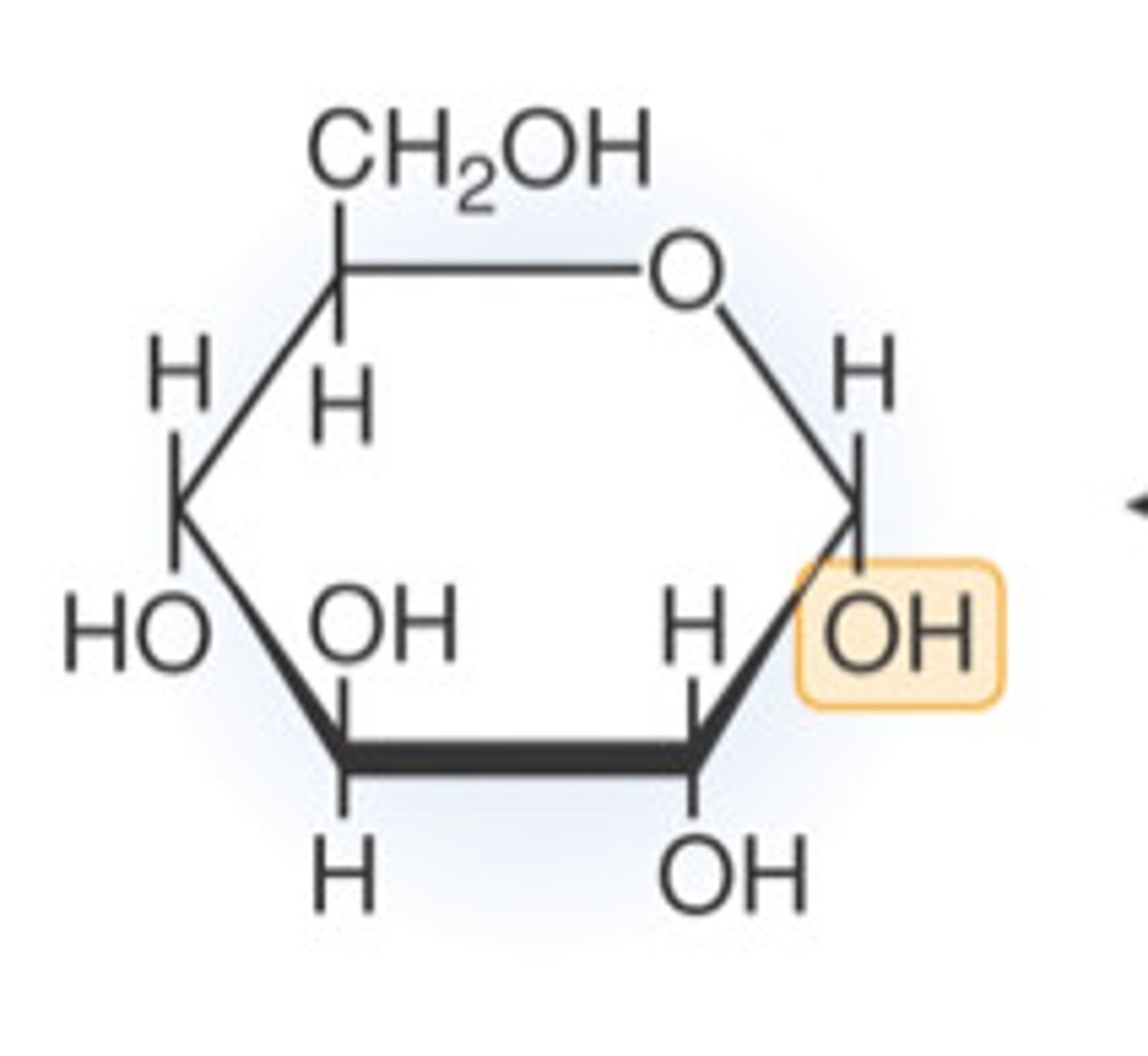
Explain the structure of ⍺-D-glucose
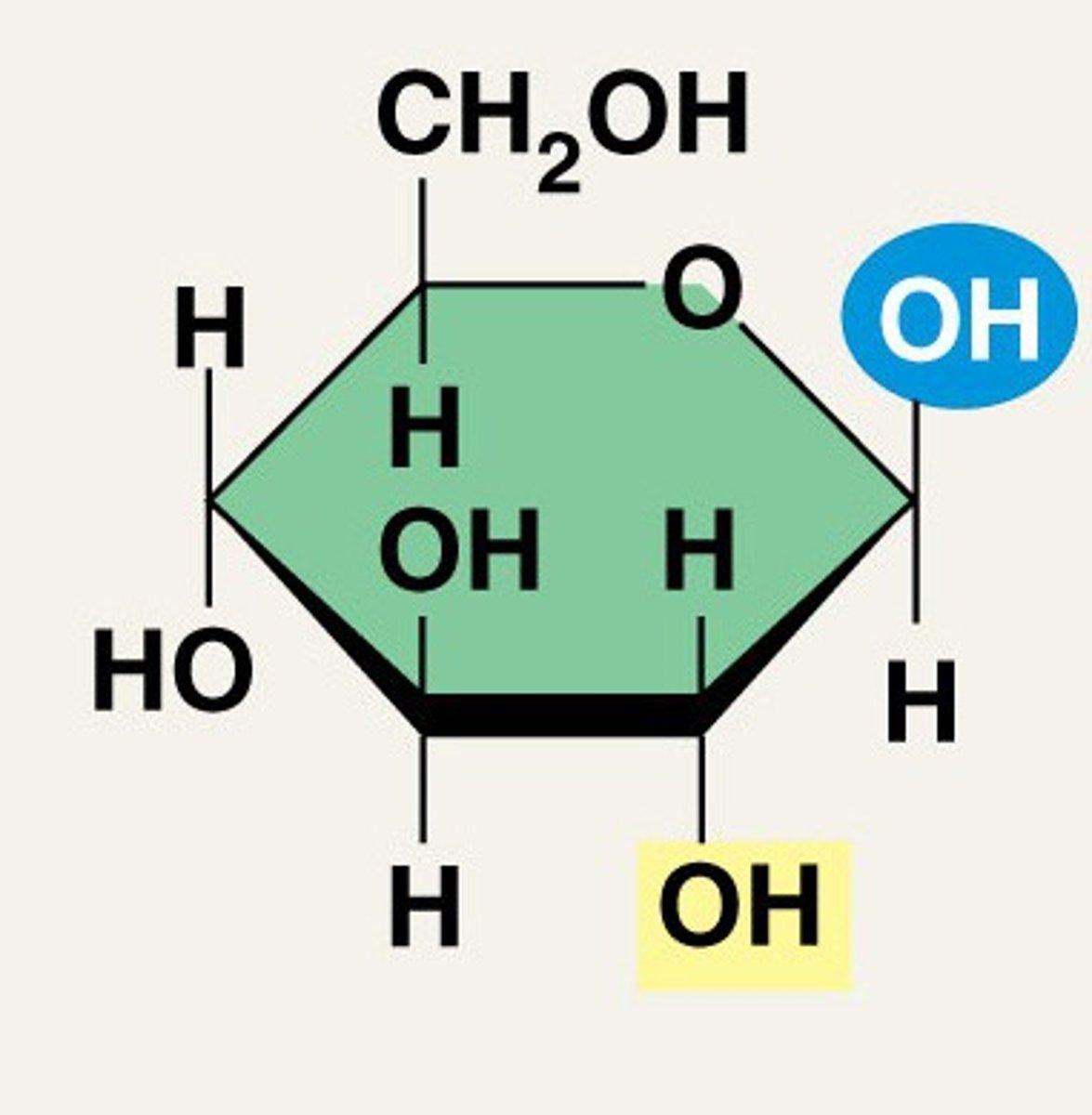
Explain the structure of β-D-glucose
Explain the structure of ribose
Disaccharides
covalent bonds between 2 monosaccharides
formula=C12H22O11 (because a water molecule is lost in dehydration synthesis)
Examples:
-sucrose=glucose and fructose (transported by phloem in plants)
-maltose=glucose and glucose
-lactose=(milk sugar) glucose and galactose
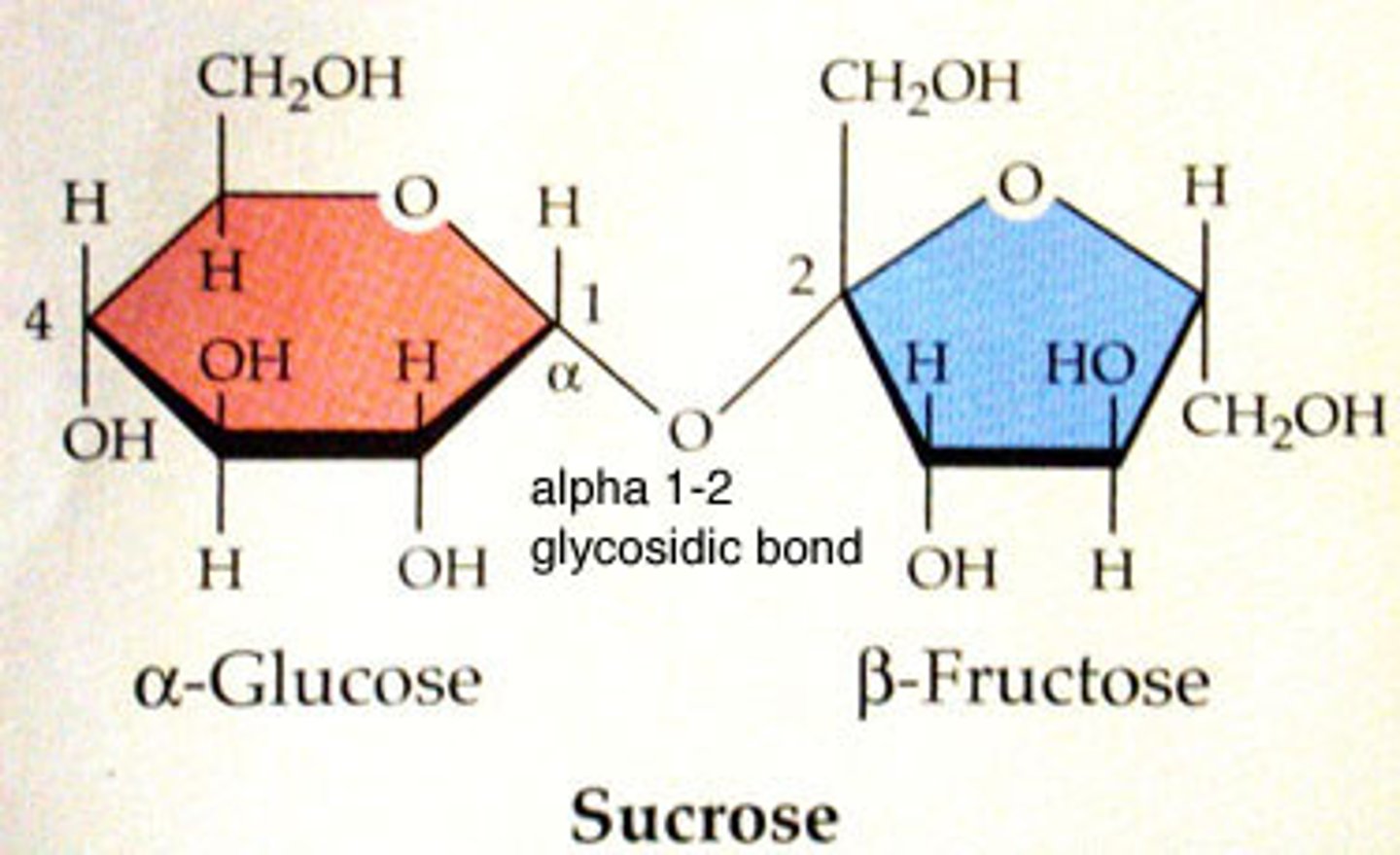
Polysaccharides
Multiple monosaccharides bonded covalently
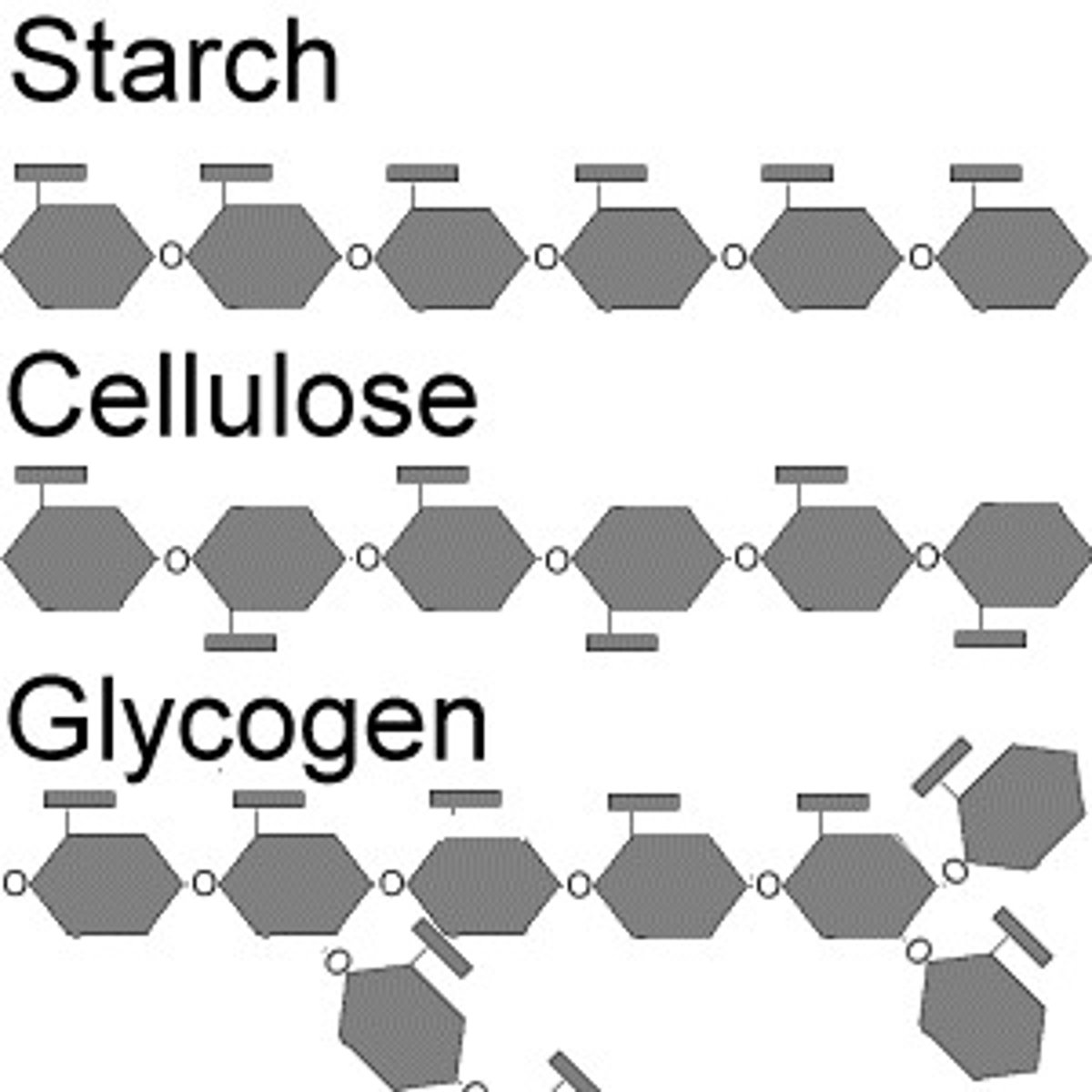
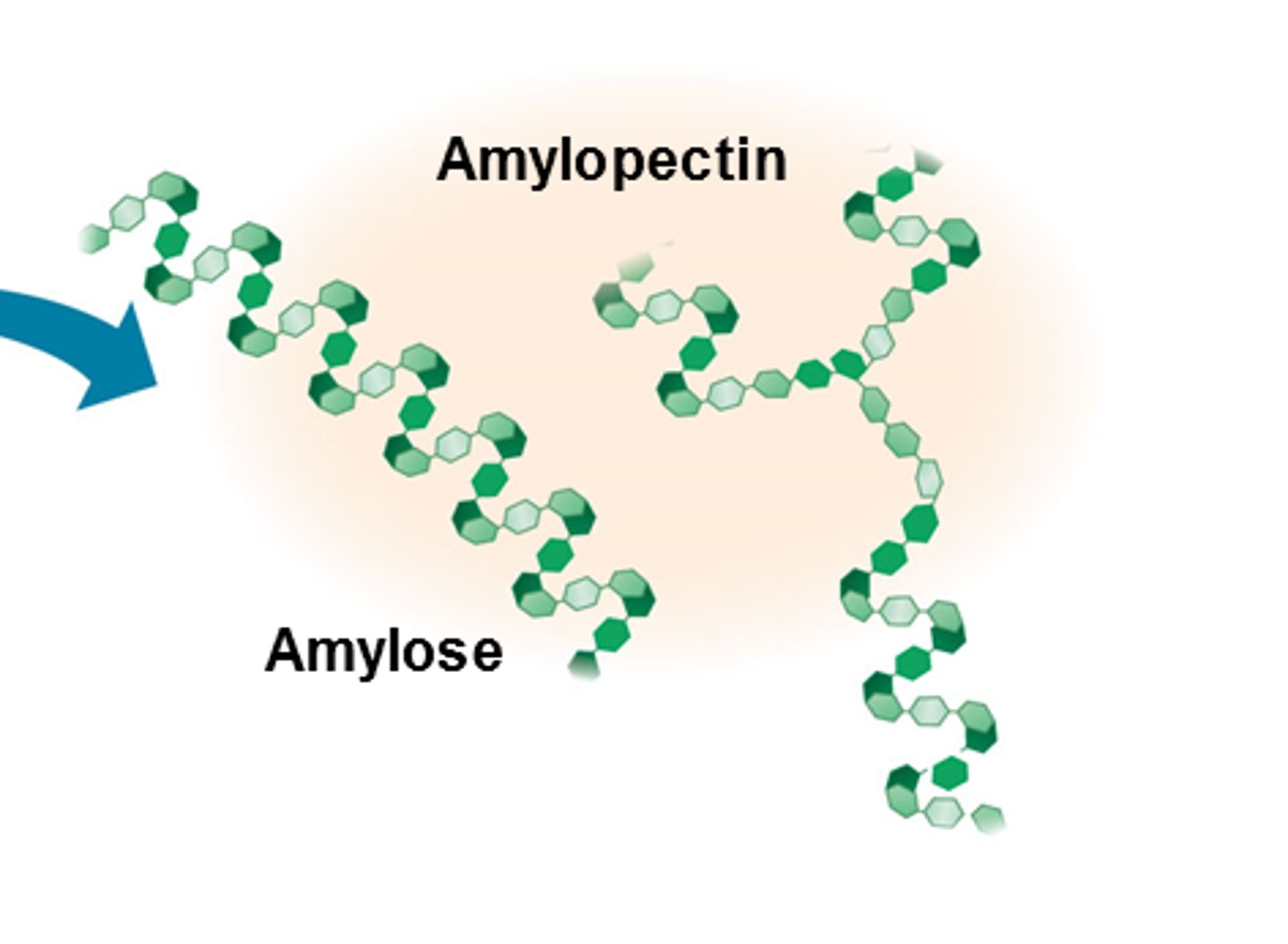
What is the function, structure, and importance of structure and function together of starch?
Function: energy storage in plants
Structure: chain of alpha glucose with same bond orientation
Amylose=unbranched
Amylopectin=branched (though less than glycogen)
F&S: flat, too big to dissolve
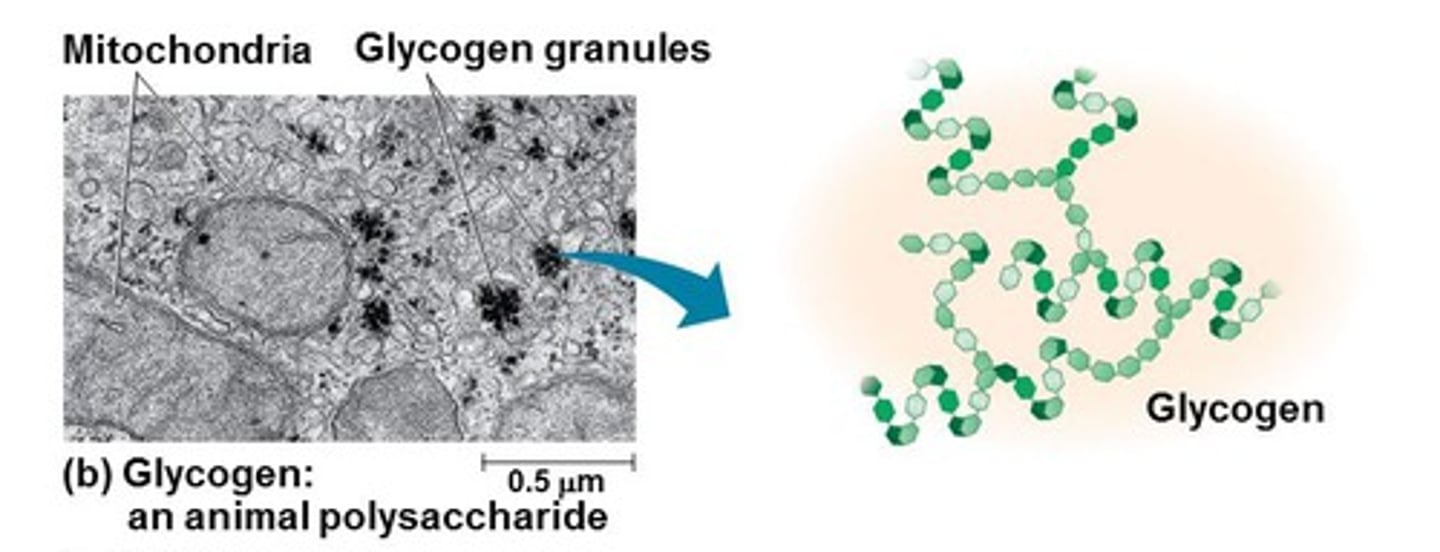
What is the function, structure, and importance of structure and function together of glycogen?
Function: energy storage in animals (esp. in liver and muscles)
Structure: more highly branched alpha glucose
F&S: quick release needed, must be branched
What is the function, structure, and importance of structure and function together of cellulose?
Function: structural material (plant cell walls)
Structure: chain of beta glucose with alternating bond orientations, gives strength (to build strong cell walls)
F&S: stacked, linear model, stronger, not soluble, good for structure, not digestable

Lipids
Fats, phospholipids, steroids, waxes
All lipids are hydrophobic
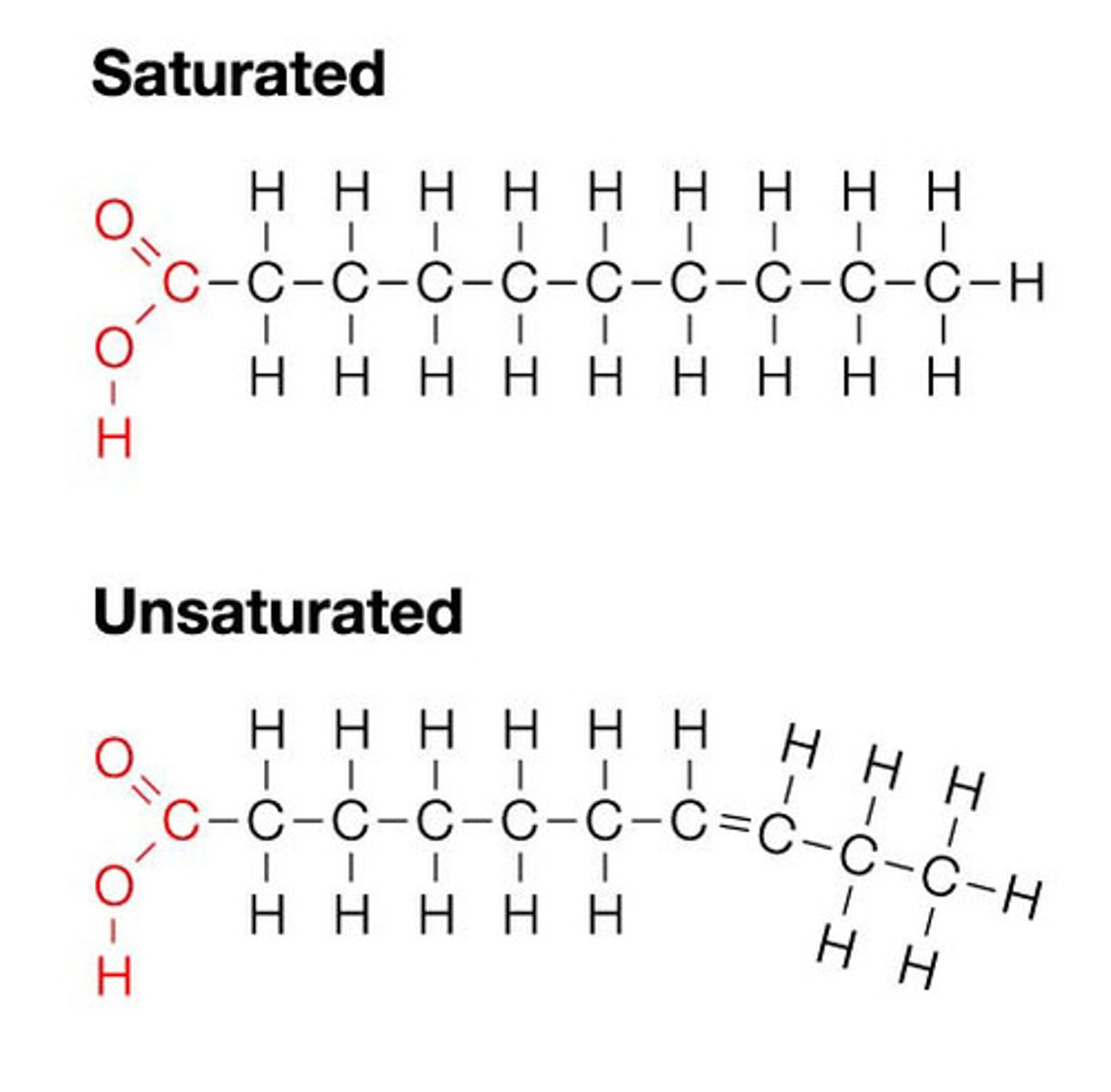
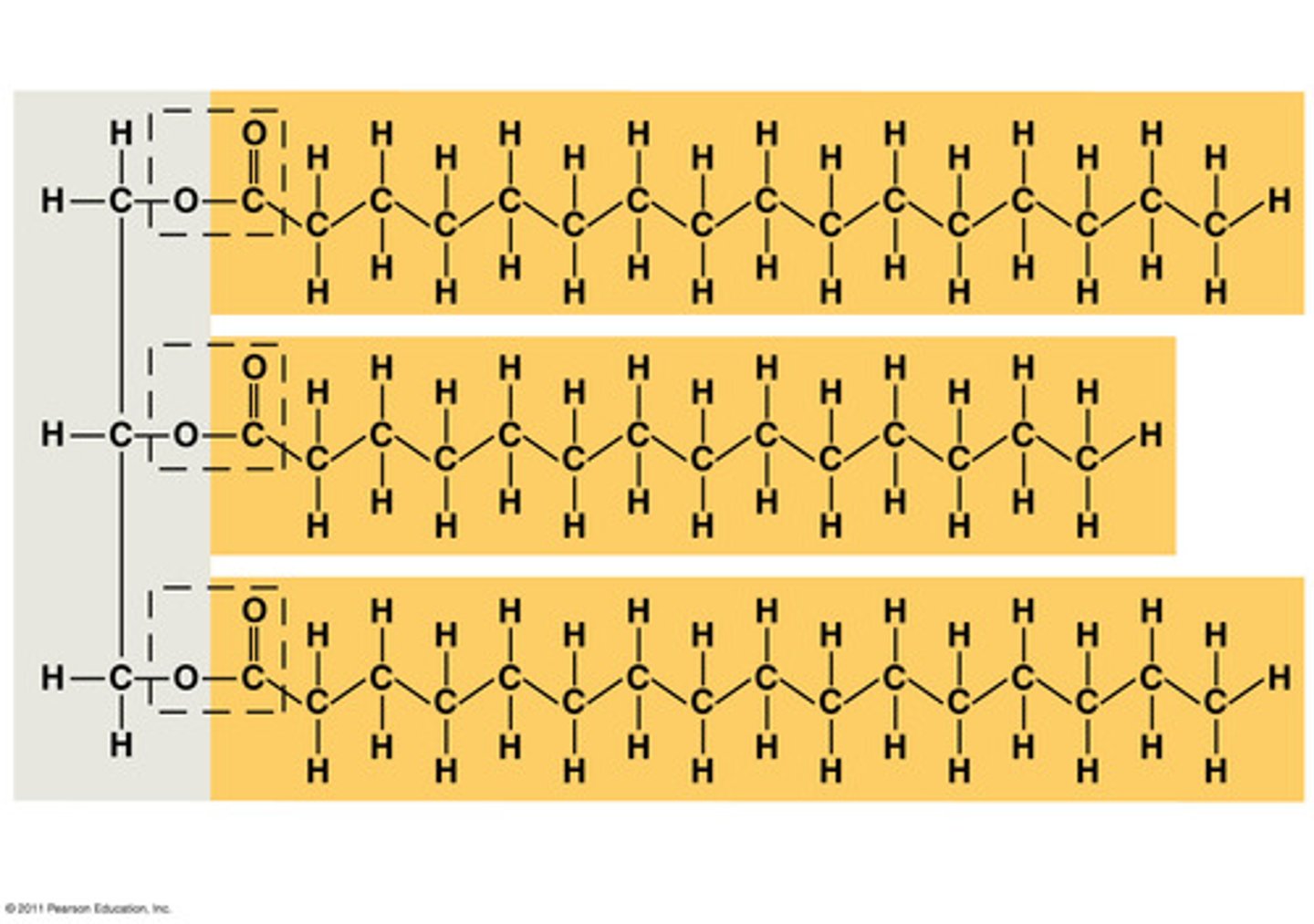
What is the structure of Fats/Triglycerides?
1 glycerol condensed with 3 fatty acids nonpolar C-H bonds in fatty acid "tails"
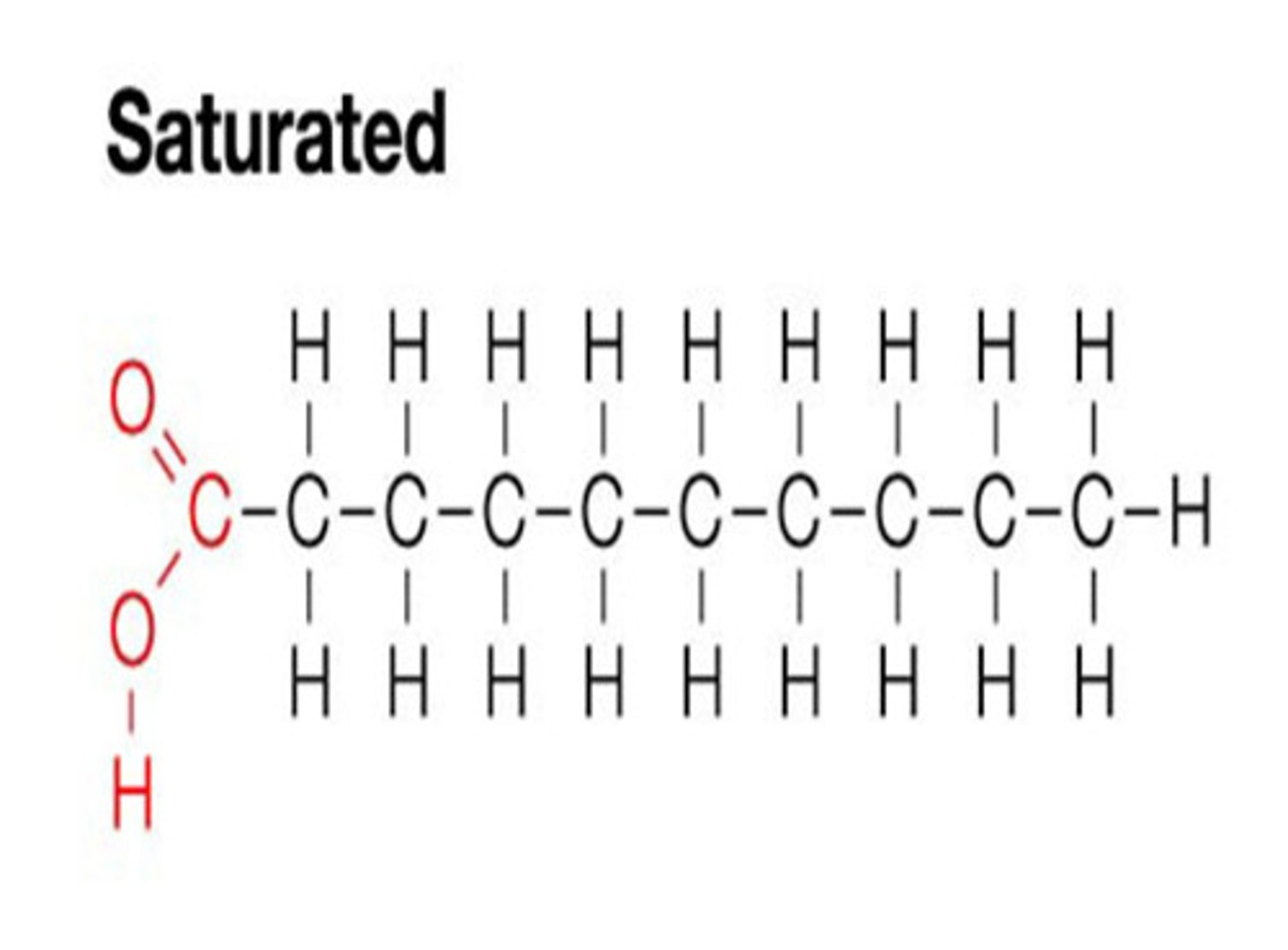
Saturated fat
single bonds between all carbons in fatty acid (i.e. saturated with hydrogen), solid at room temperature

Unsaturated fat (oils)
contain double bonds between carbons in fatty acid

What are the different types of unsaturated fats?
Monounsaturated, polyunsaturated, cis, and trans
Monounsaturated fats
have only one double bond

Polyunsaturated fats
more than one double bond

Cis fats
hydrogen atoms on same side of double bond (typical natural isomer bend in fatty acid), liquid at room temperature
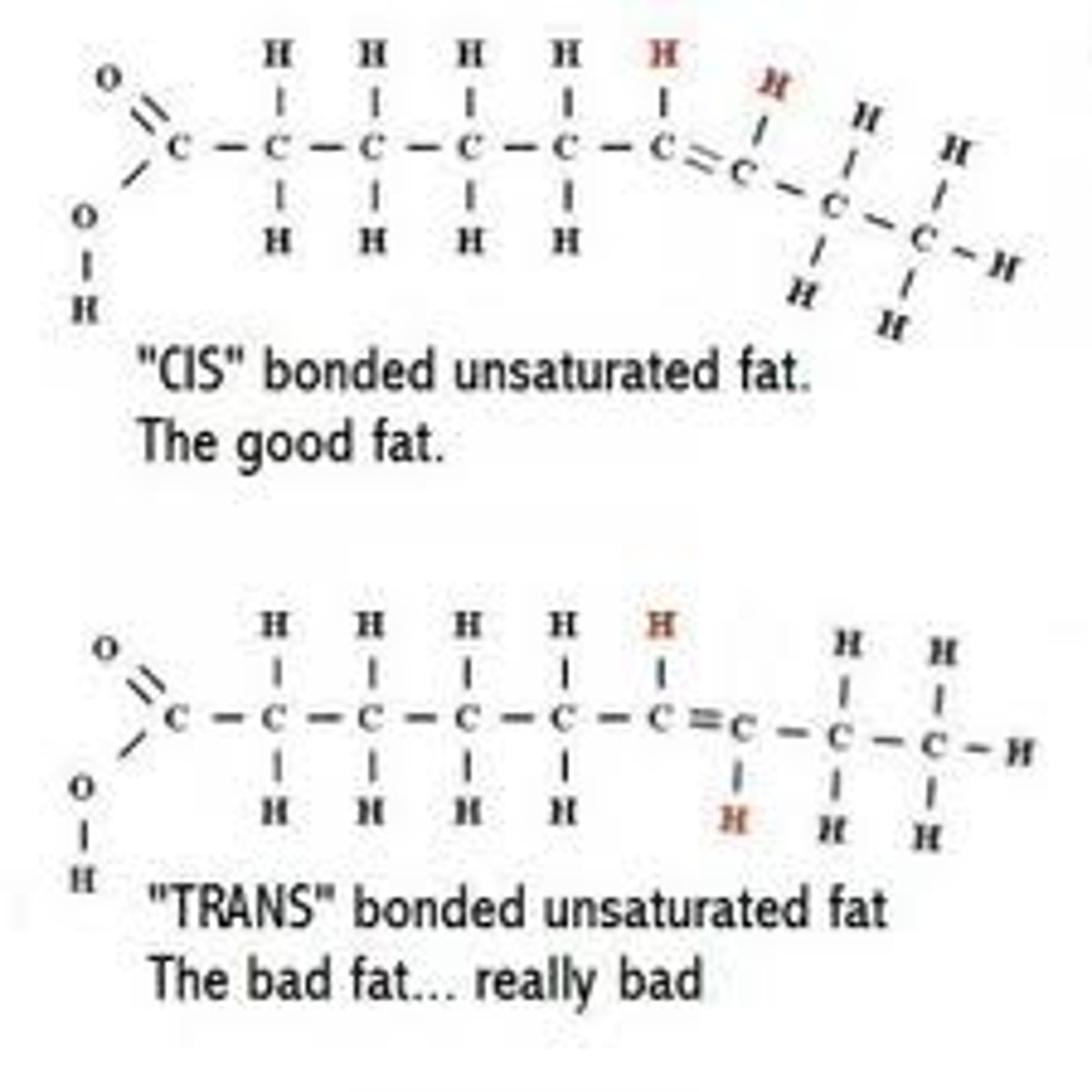
Trans fats
hydrogen atoms on opposite sides of the double bond (produced by artificial partial hydrogenation of oils, no bend in fatty acid), solid at room temperature

What is the general formula for saturated fats?
COOH-(CH2)n-CH3
List the purposes of fats
energy storage, organ cushioning, and thermal insulation
List the purposes of carbohydrates
short terms storage, easier to digest (more rapid energy release), soluble in water (easier to transport in blood etc.), 4 calories/gram
List the purposes of lipids
long term storage, more energy/gram (lighter energy storage), insoluble in water so they don't cause problems with osmosis
Body Mass Index (BMI)
BMI=(weight in kg)/(height in m^2)
metric

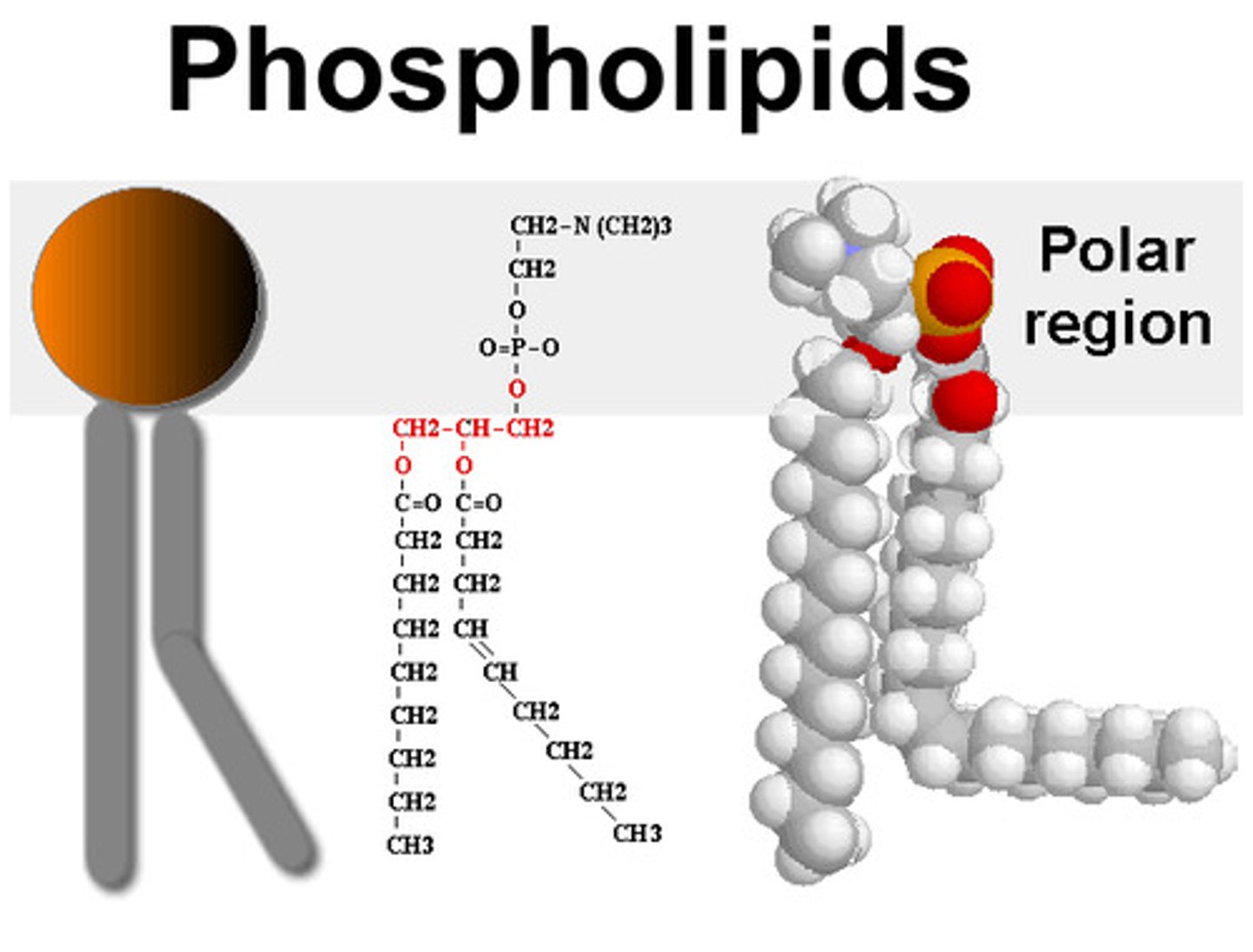
Phospholipids
structure same as fat except 2 fatty acid tails and a phosphate group attached to glycerol
Steroids
lipids with 4 fused carbon rings
Example: Cholesterol
-used in cell membranes
-precursor for other steroids (sex hormones)
-atherosclerosis

Proteins
wide range of functions, each has a complex 3-D shape (conformation), monomers are amino acids (there are 20)
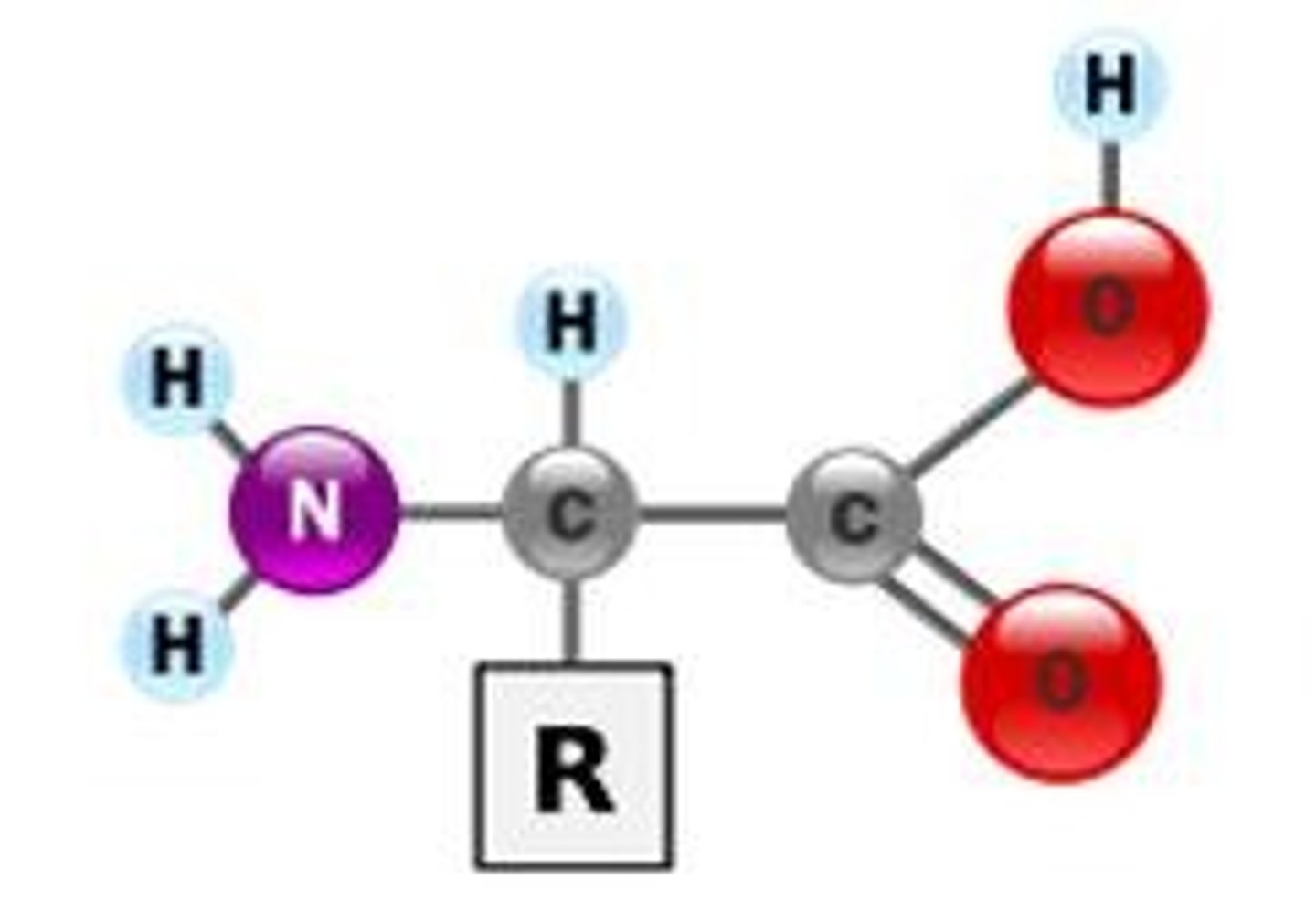
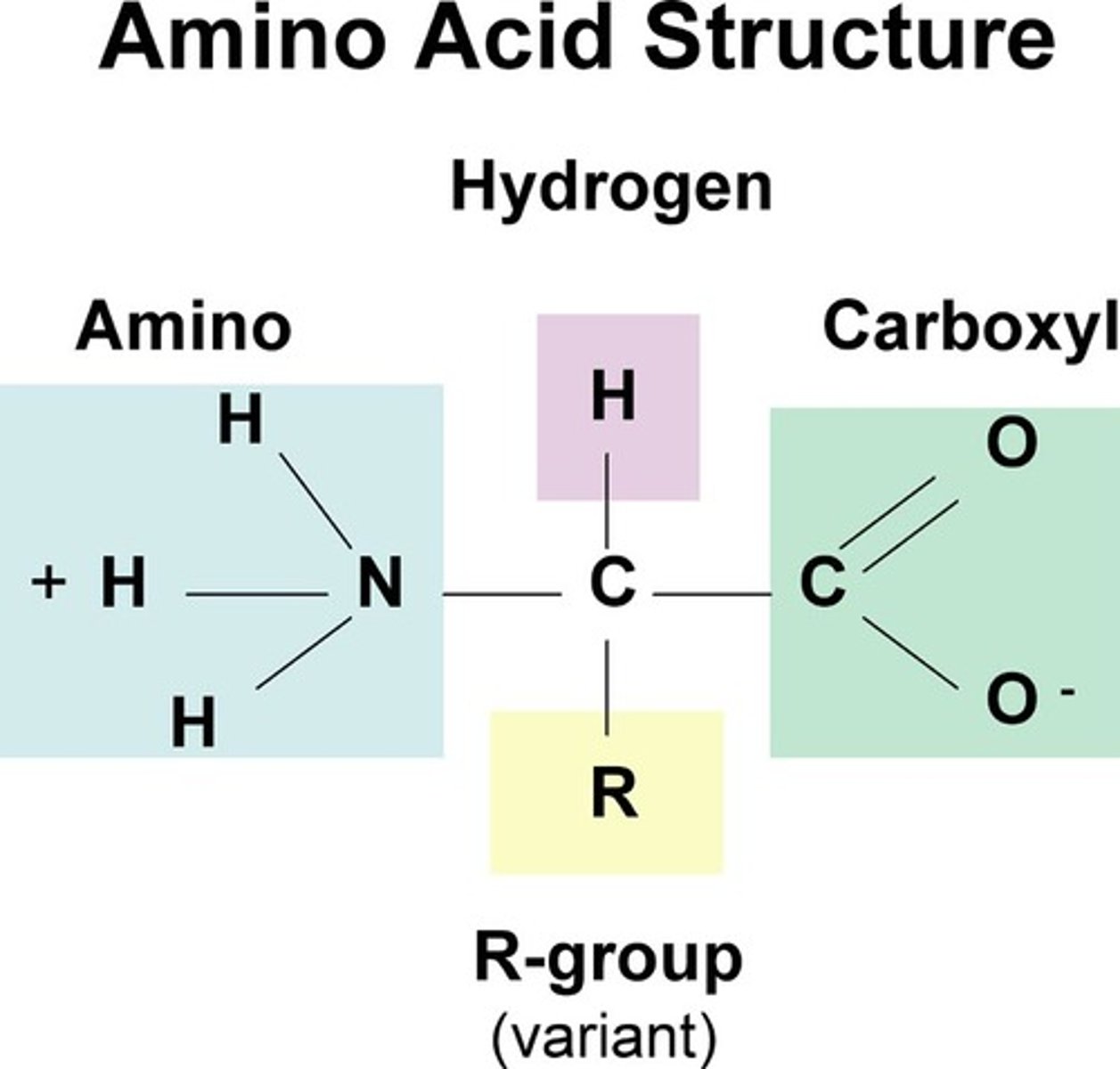
What are the four parts surrounding a central carbon in an amino acid?
1) amino group (NH2)
2) carboxyl group (-COOH)
3) H atom
4) variable groups (determine the amino acid's properties, ex. polar (hydrophilic), nonpolar (hydrophobic), acid, or base)
The only part that varies is the R-group
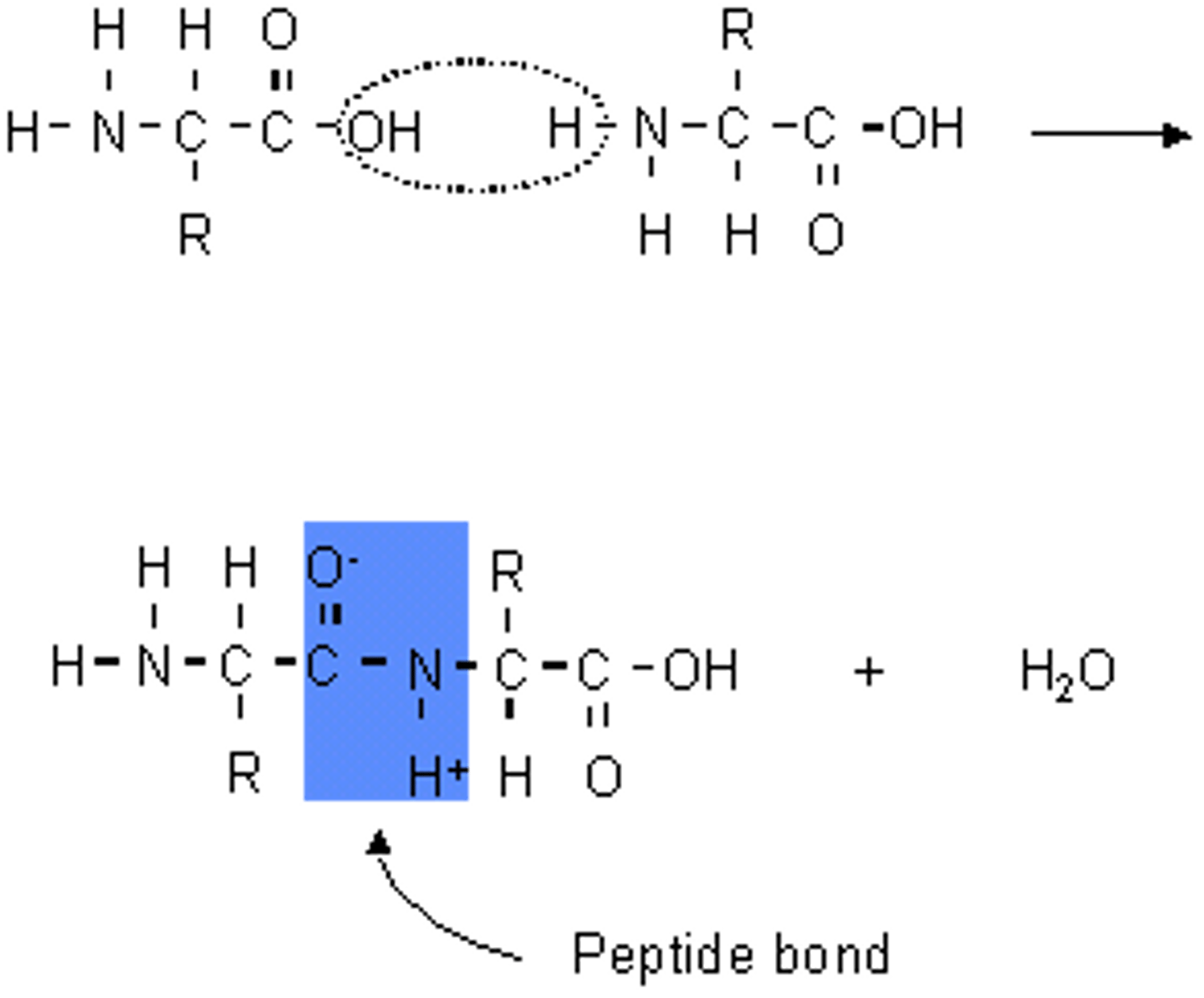
Polypeptides
formed by condensation reaction, multiple amino acids
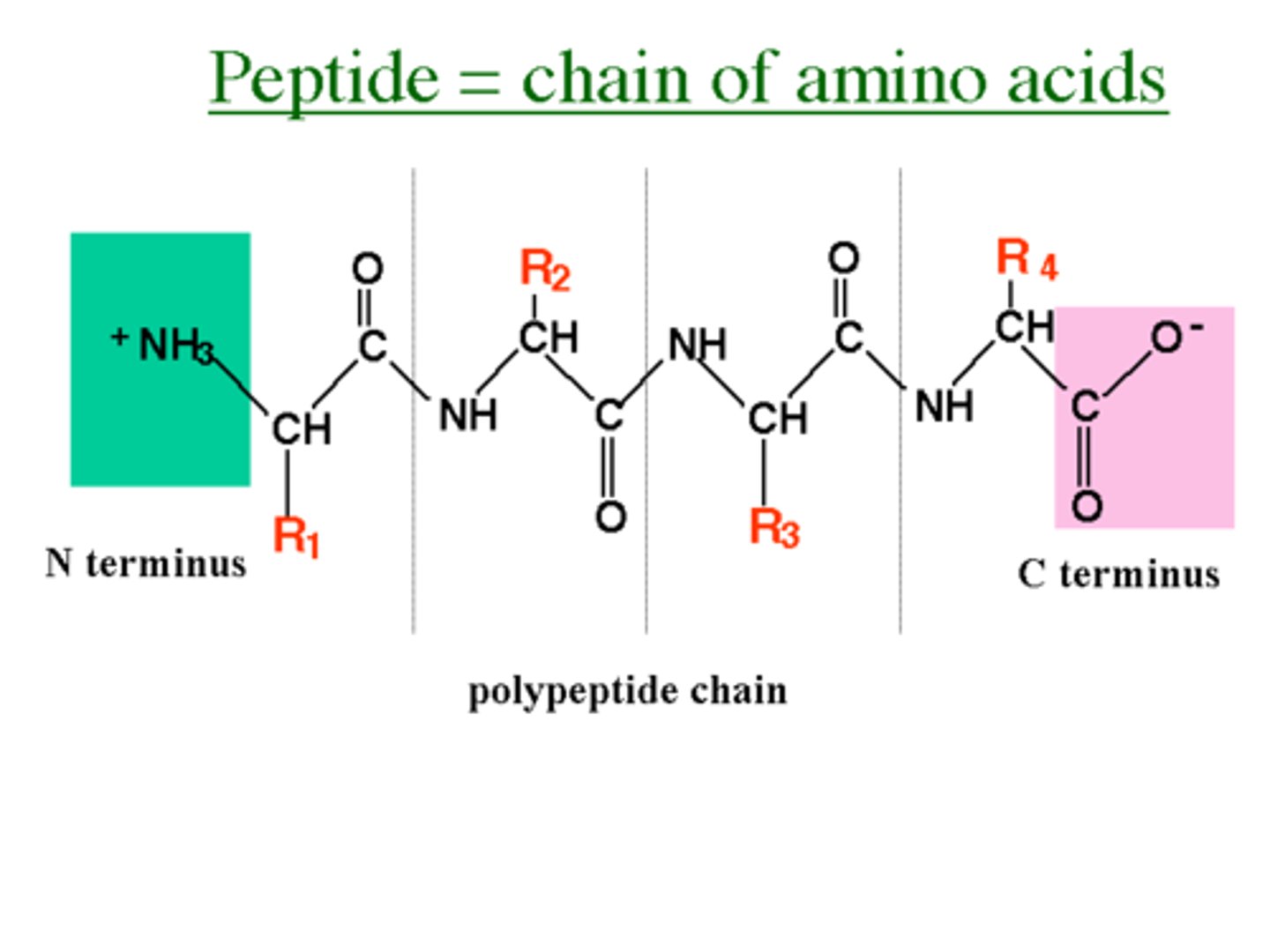
Peptide bonds
covalent bonds (carboxyl group to amino group)
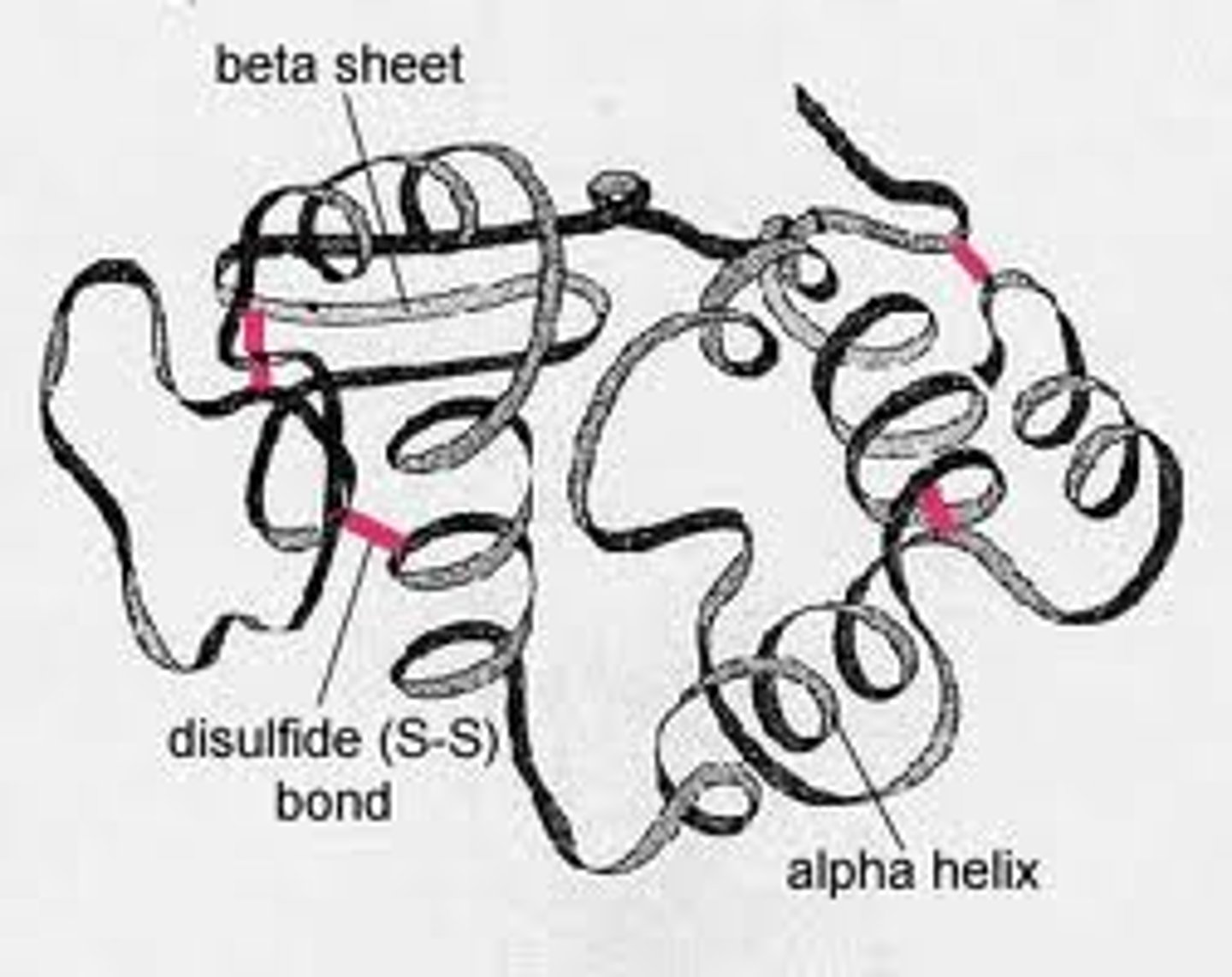
What are the four levels of structure in a protein?
Primary, secondary, tertiary, and quaternary
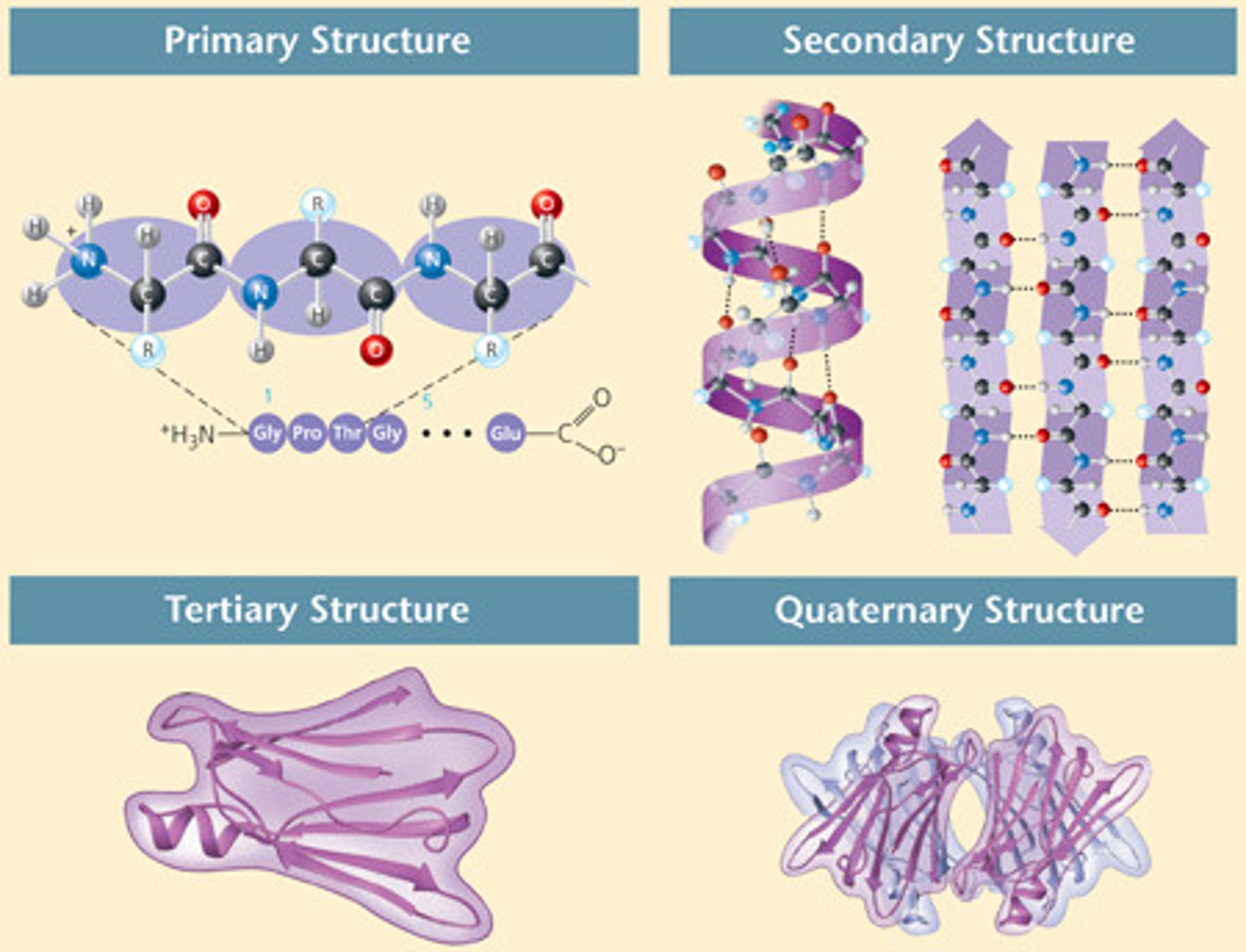
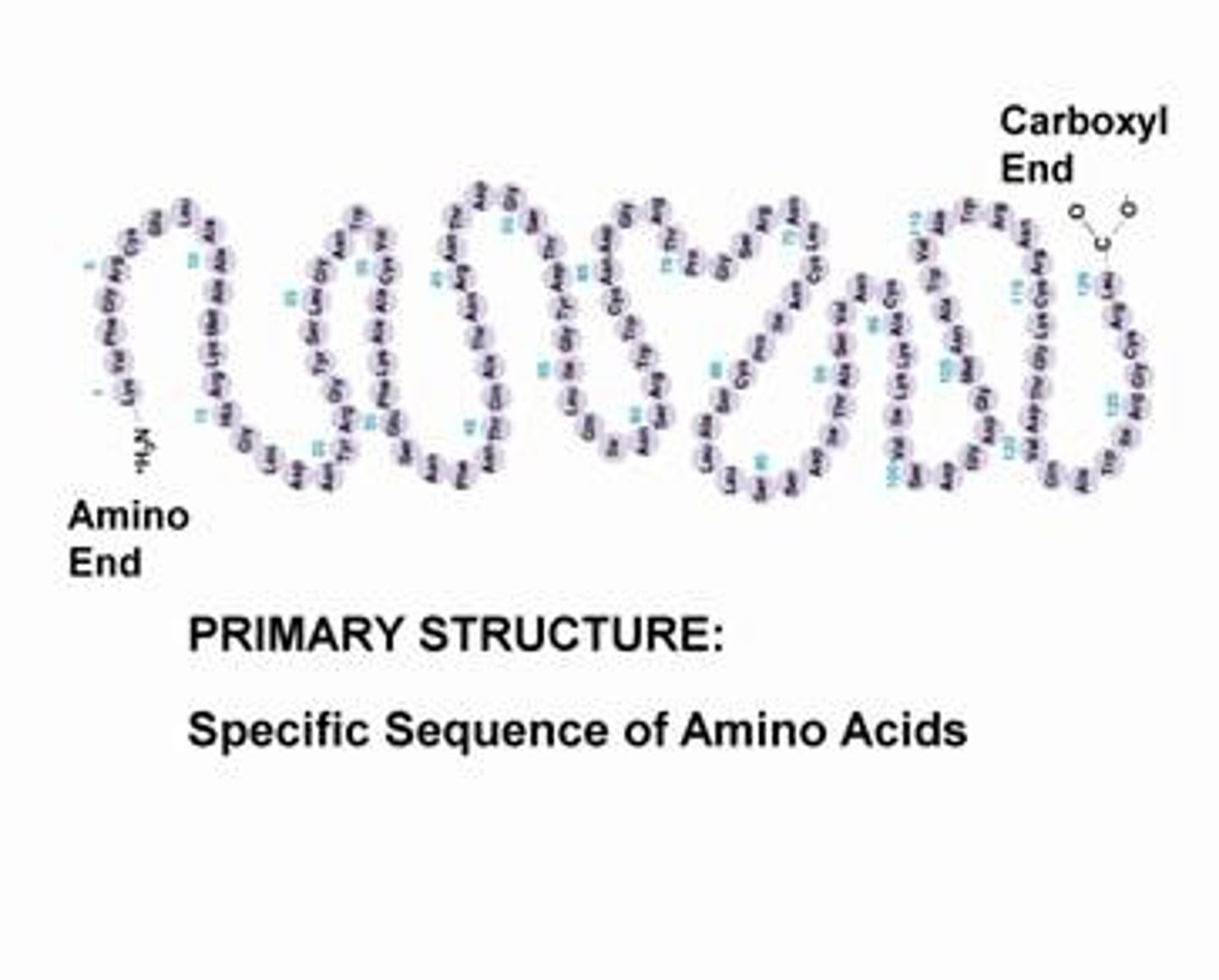
Primary structure
Order of amino acids, each type of protein has a unique primary structure, sequence determines 3D conformation
Amino acid substitution: hemoglobin, sickle-cell anemia
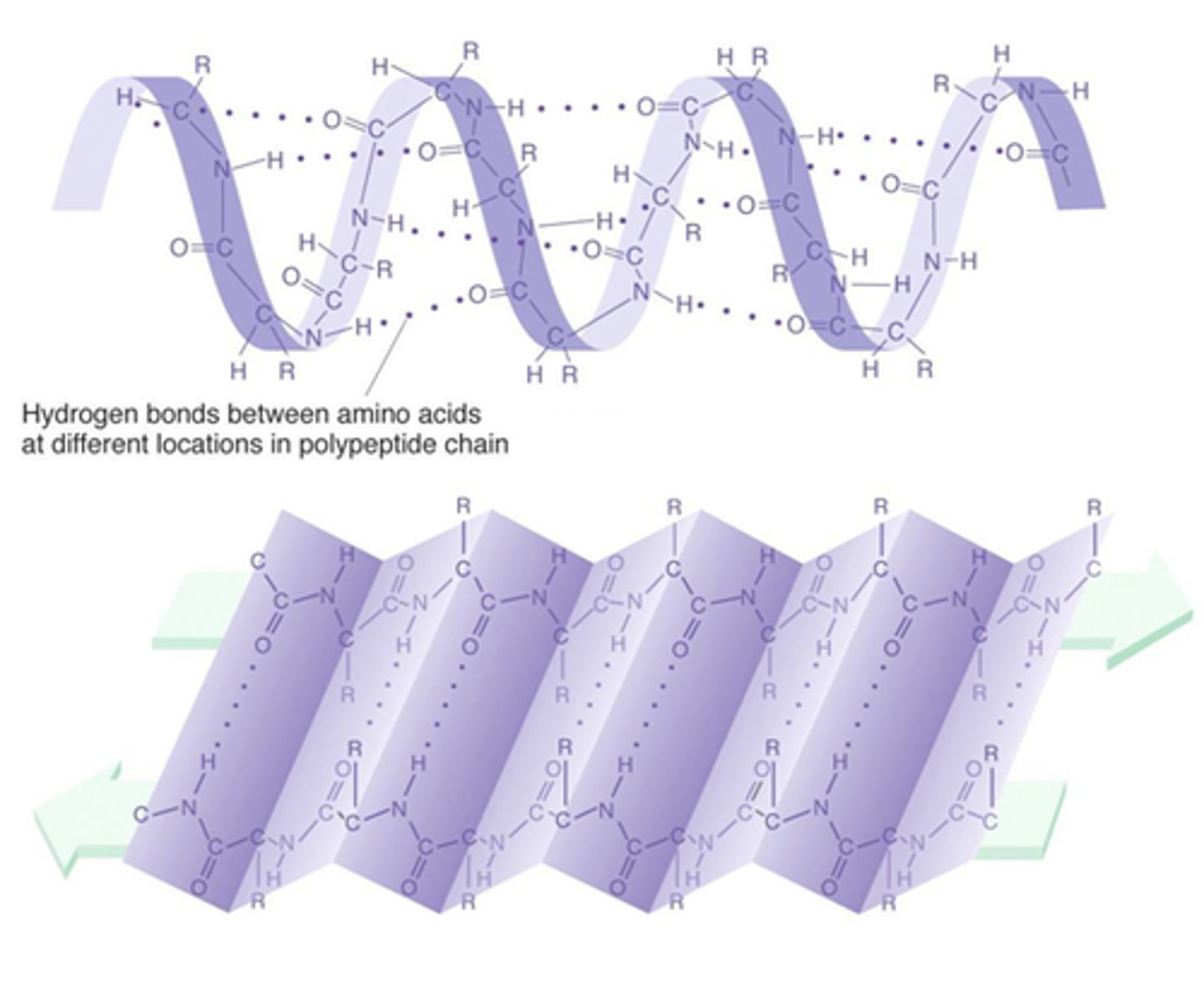
Secondary structure
Coils and foils (due to hydrogen bonds at regular intervals), Alpha helix causes coiling (ex. keratin), pleated sheet is parallel (ex. silk)
Tertiary structure
Overall 3D shape of the polypeptide
Contortions from R-group bonding
-hydrophobic interactions (nonpolar side chains)
-disulfide bridges (strong covalent bonds)
-hydrogen bonds (between polar side chains)
-ionic bonds (charged side chains)
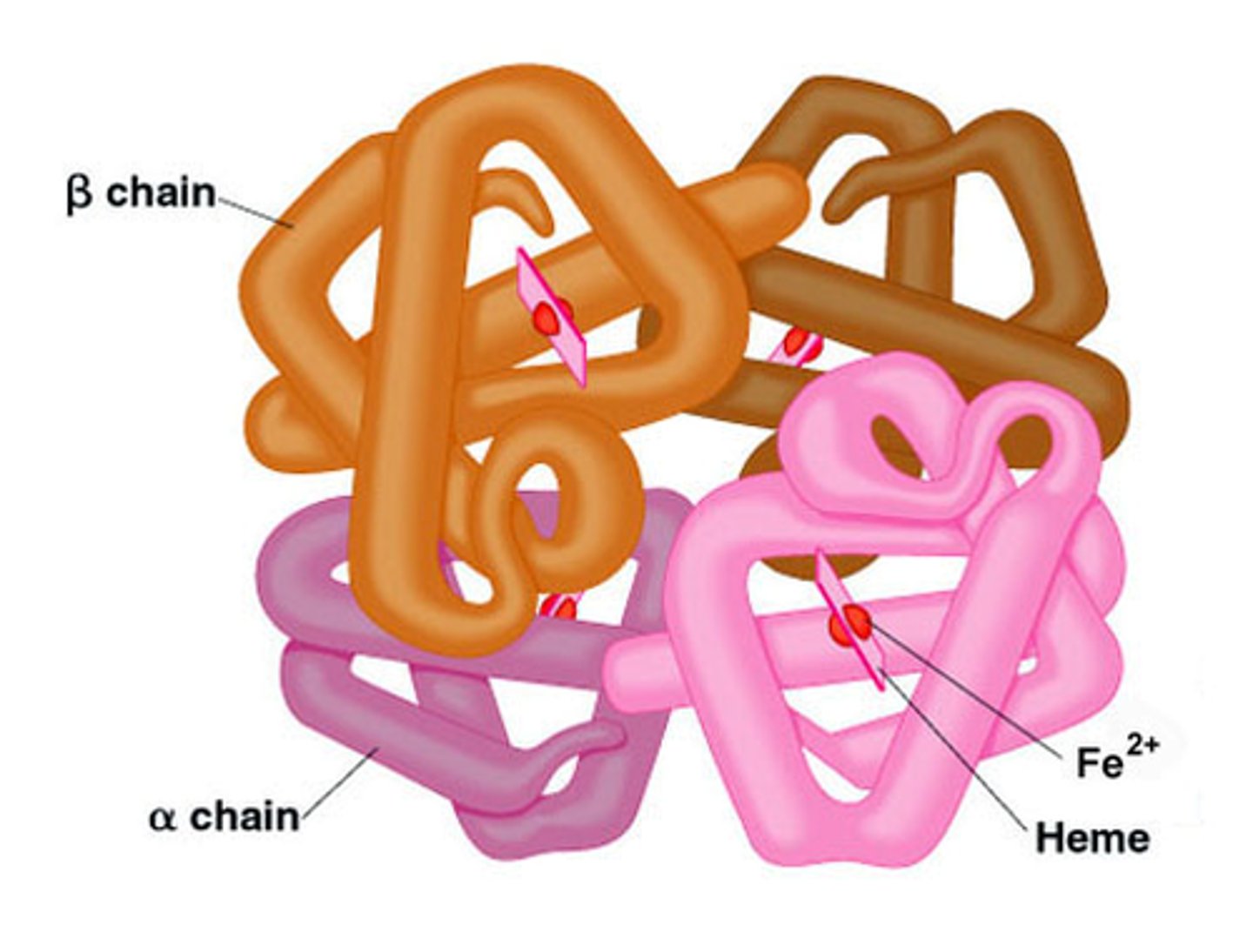
Quaternary structure
2 or more polypeptide chains aggregated into 1 macromolecule (ex. collagen (connective tissue), hemoglobin)
List the purposes of proteins (general)
structure, transport, muscle contraction, defense, cell adhesion, tensile strengthening, DNA packaging, hormones, receptors, catalysis (enzymes), etc.
Give examples of functions of specific proteins
-Rubisco=carbon fixation during photosynthesis
-Insulin=hormone that regulates blood sugar by signaling glucose uptake by cells
-Immunoglobulins (antibodies)=specific immunity
-Rhodopsin=pigment that absorbs light in rod cells of retina
-Collagen=forms strong mesh of fibers in body (skin, blood, vessel walls, ligaments, bones, etc.)
-Spider silk=strong fibers for forming webs
Proteome
All proteins produced by a cell, tissue, or organism at any given time
Every individual has a unique proteome (exception: identical twins, but become different as they age)
Activation Energy
Amount of energy necessary to start a reaction (energy required to break bonds in reactions)
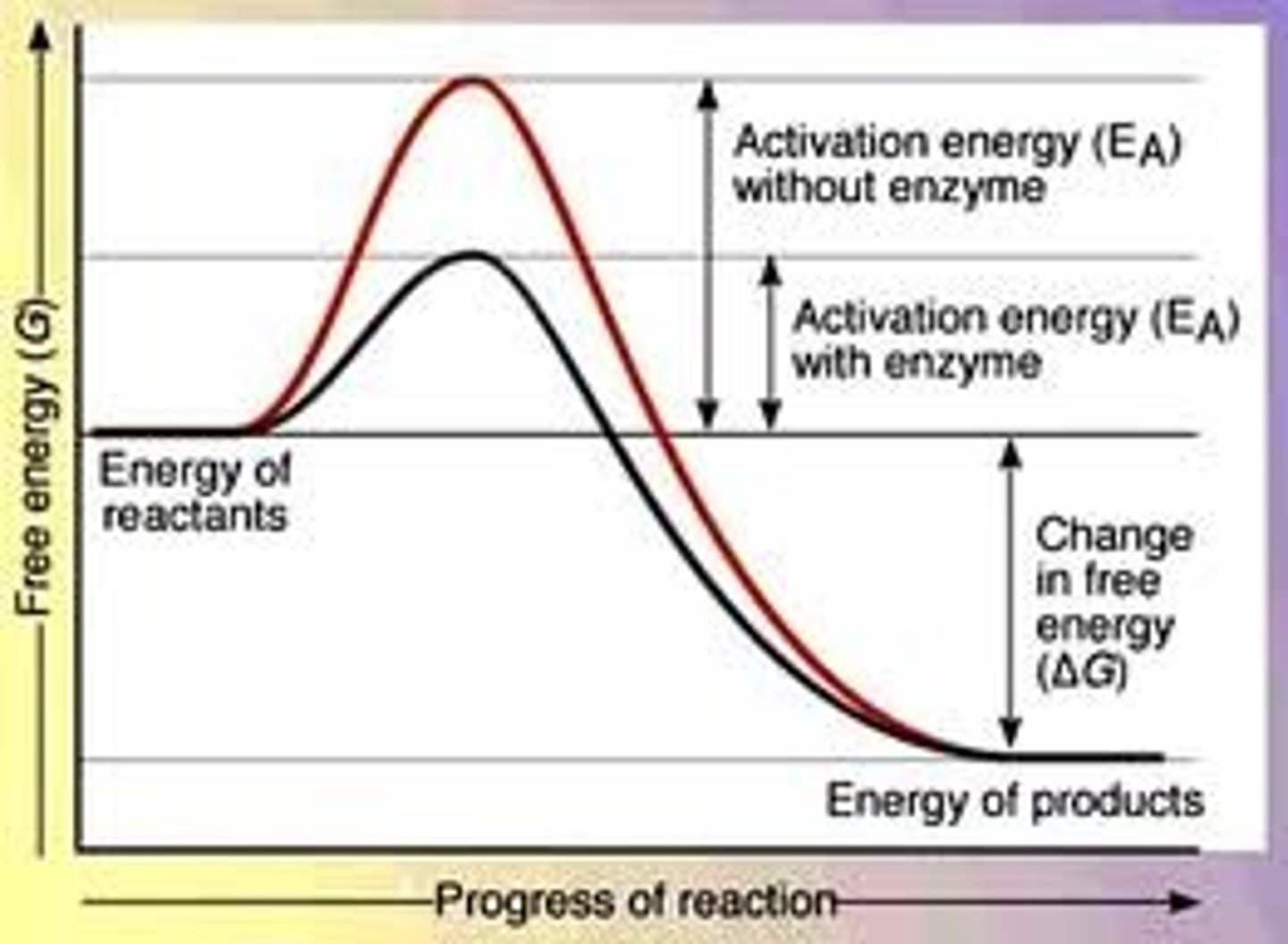
Enzymes
Catalytic proteins that change the rate of reactions w/o being consumed; they lower activation energy
Substrate
Enzyme reactant
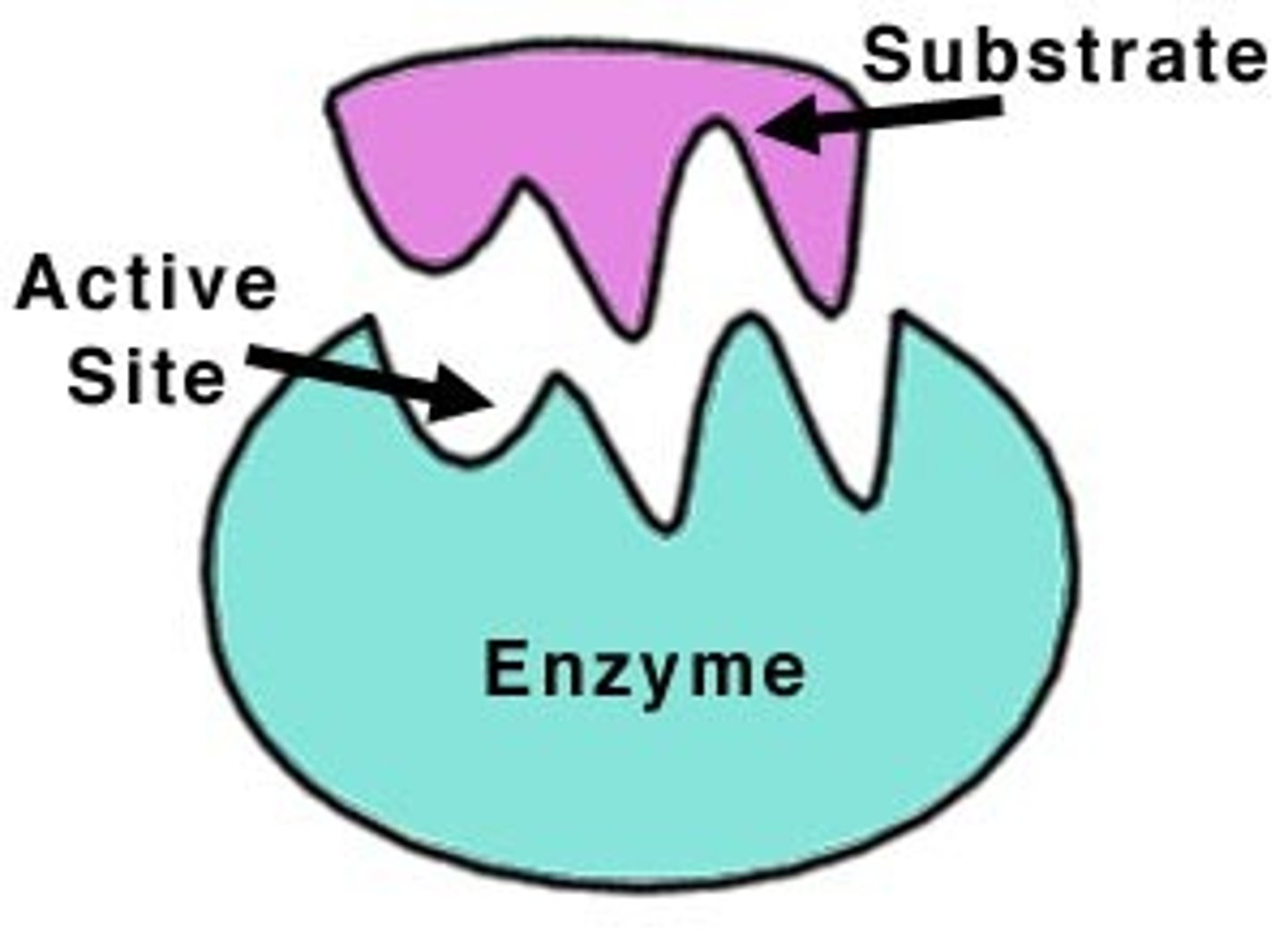
Active Site
Pocket or groove on an enzyme that binds to substrate (i.e. the spot on the enzyme where reaction occurs)
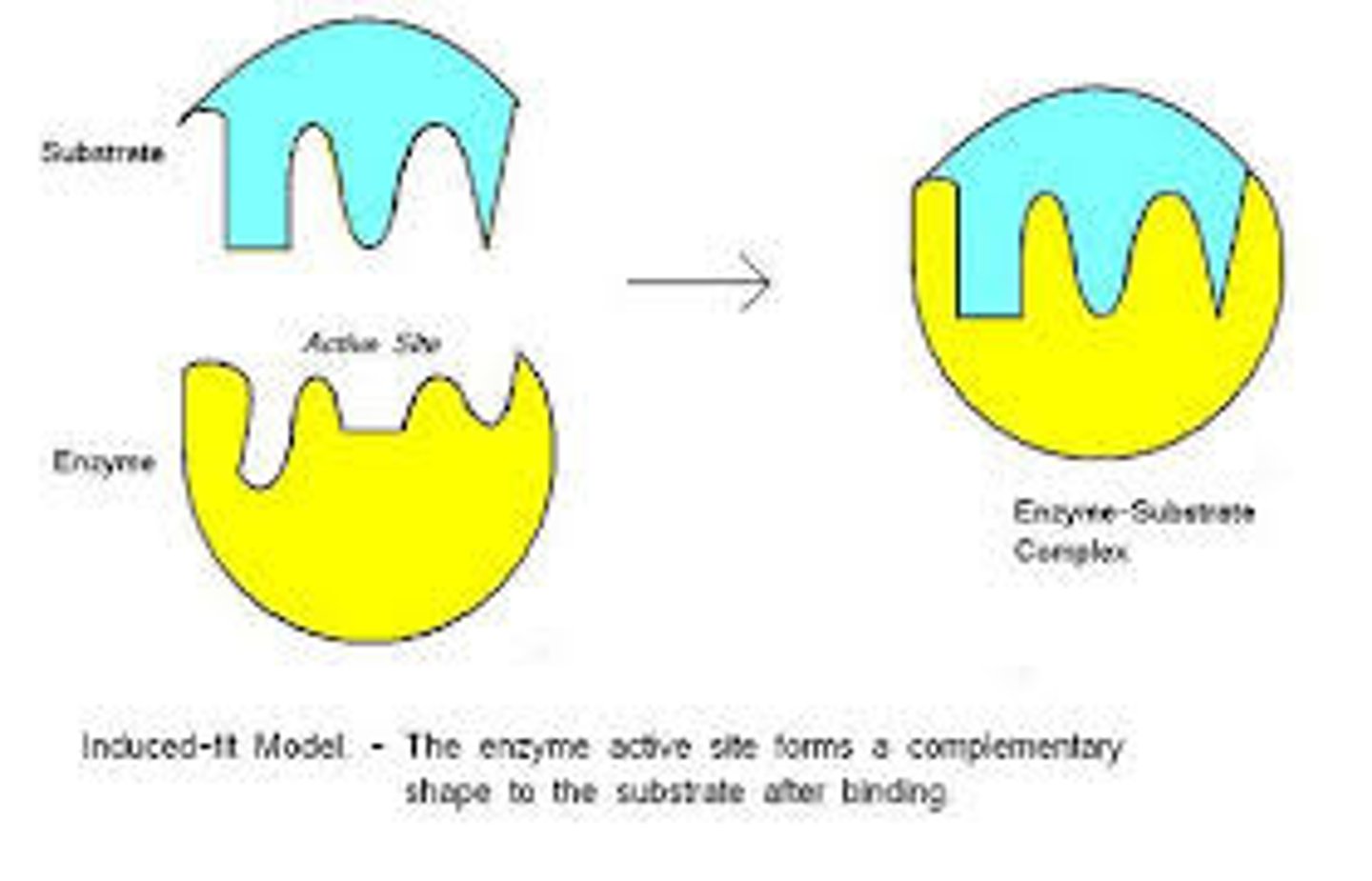
Induced fit model
Enzyme and substrate fit together, but enzyme conforms to substrate
How do enzymes control metabolic pathways?
Chains and cycle of enzyme, catalyzed reactions; ex. Cellular respiration - glycolysis: a chain, krebs cycle
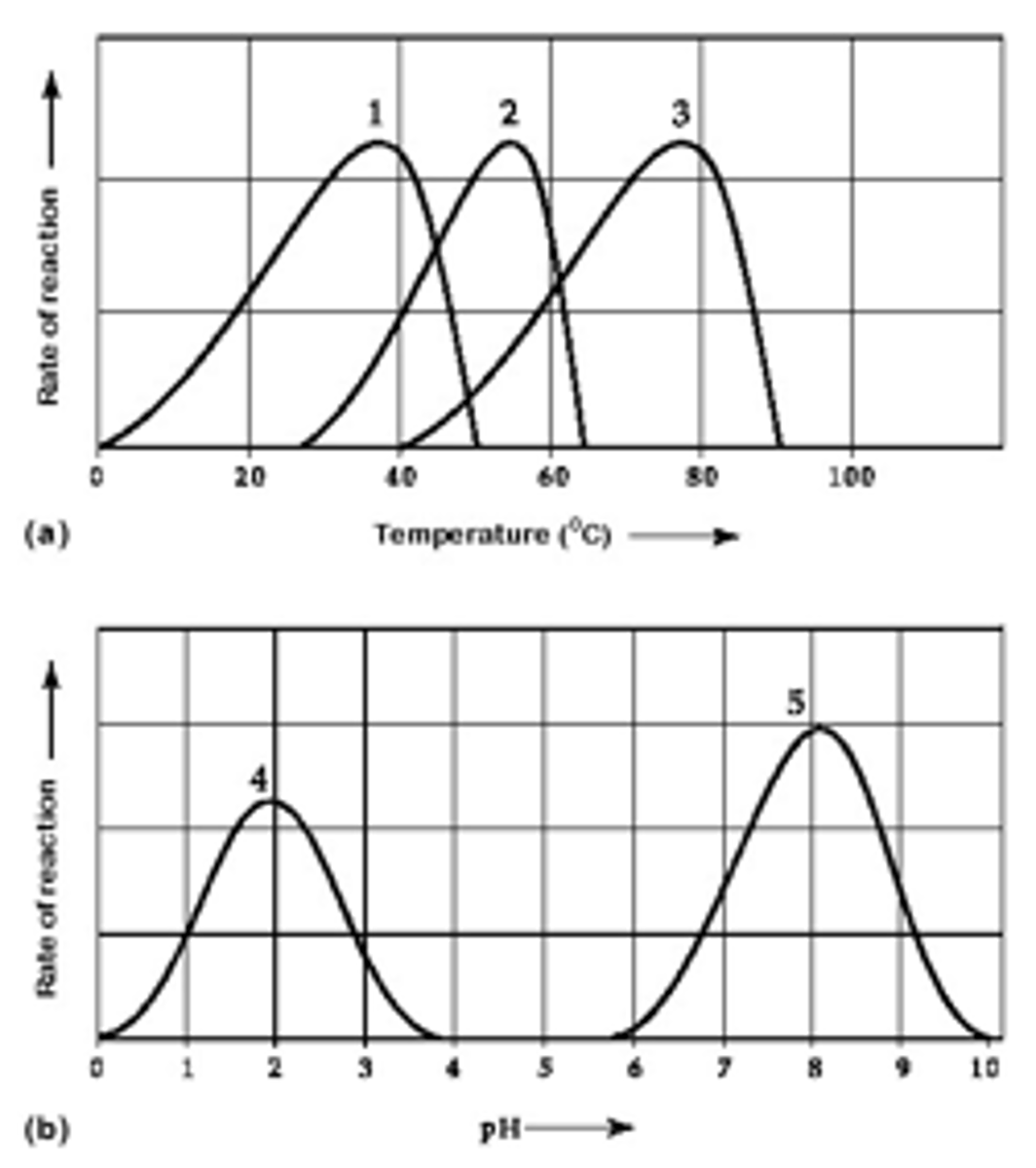
Explain the temperature graph of an enzyme
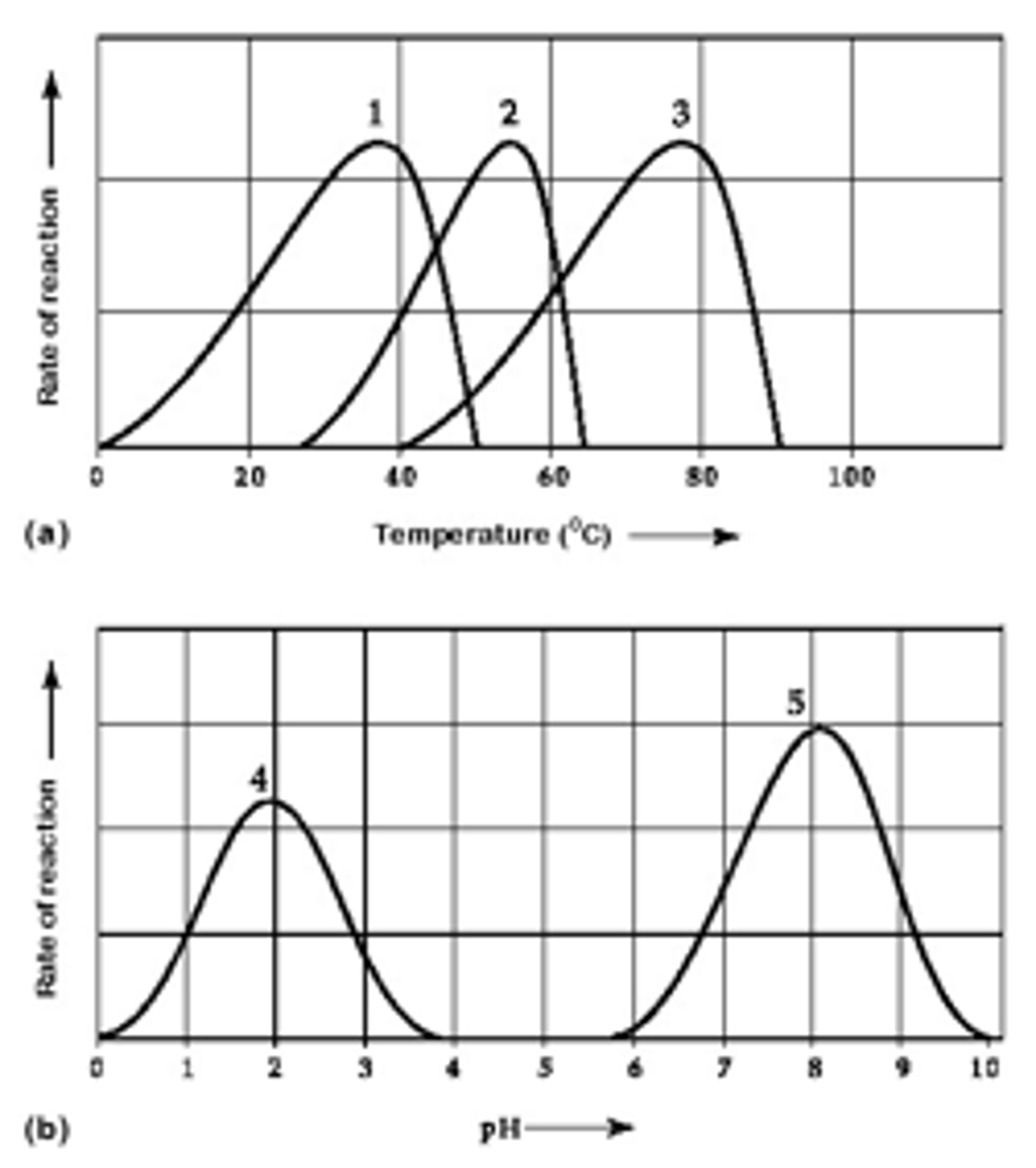
Explain the pH graph of an enzyme
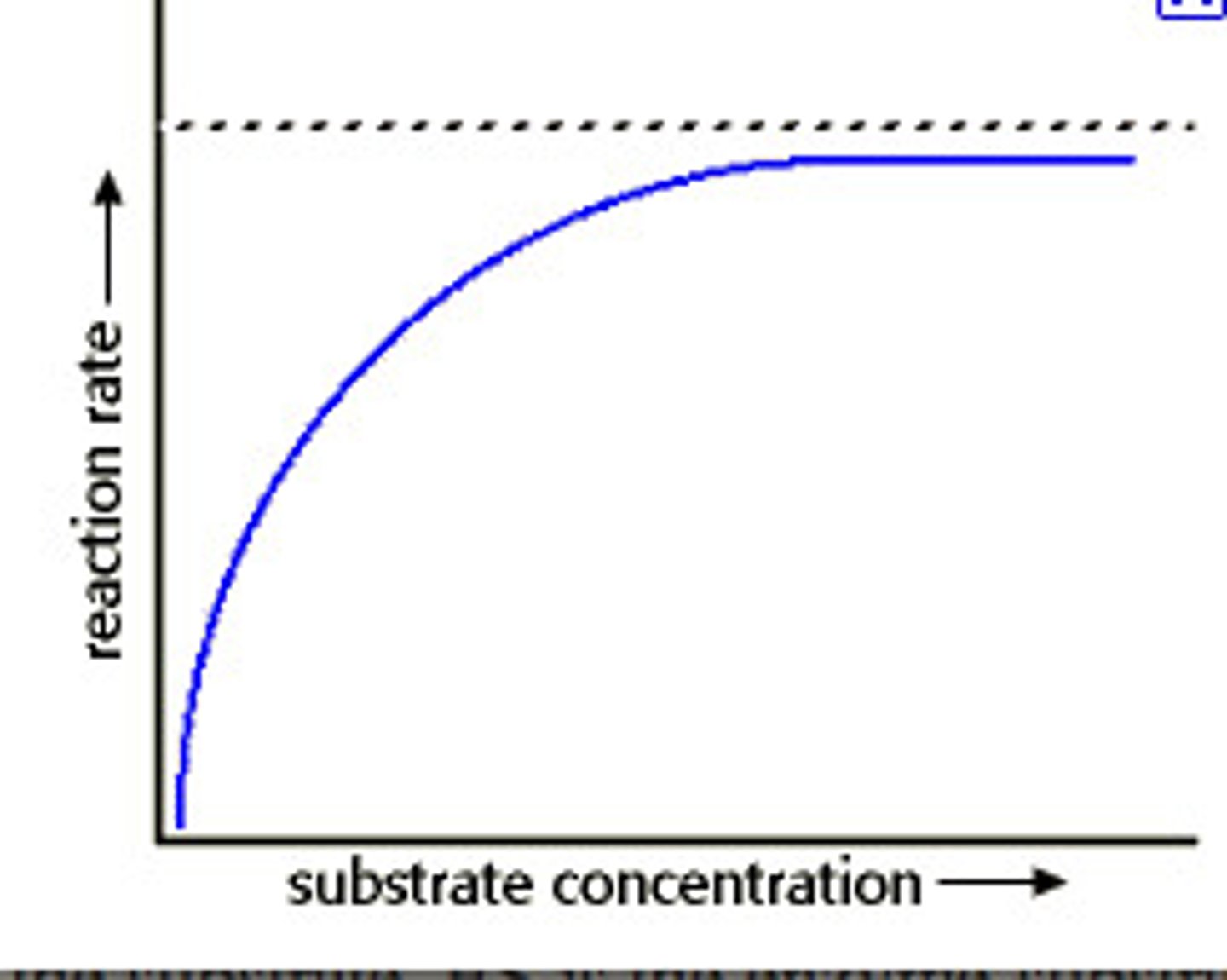
Explain the substrate graph
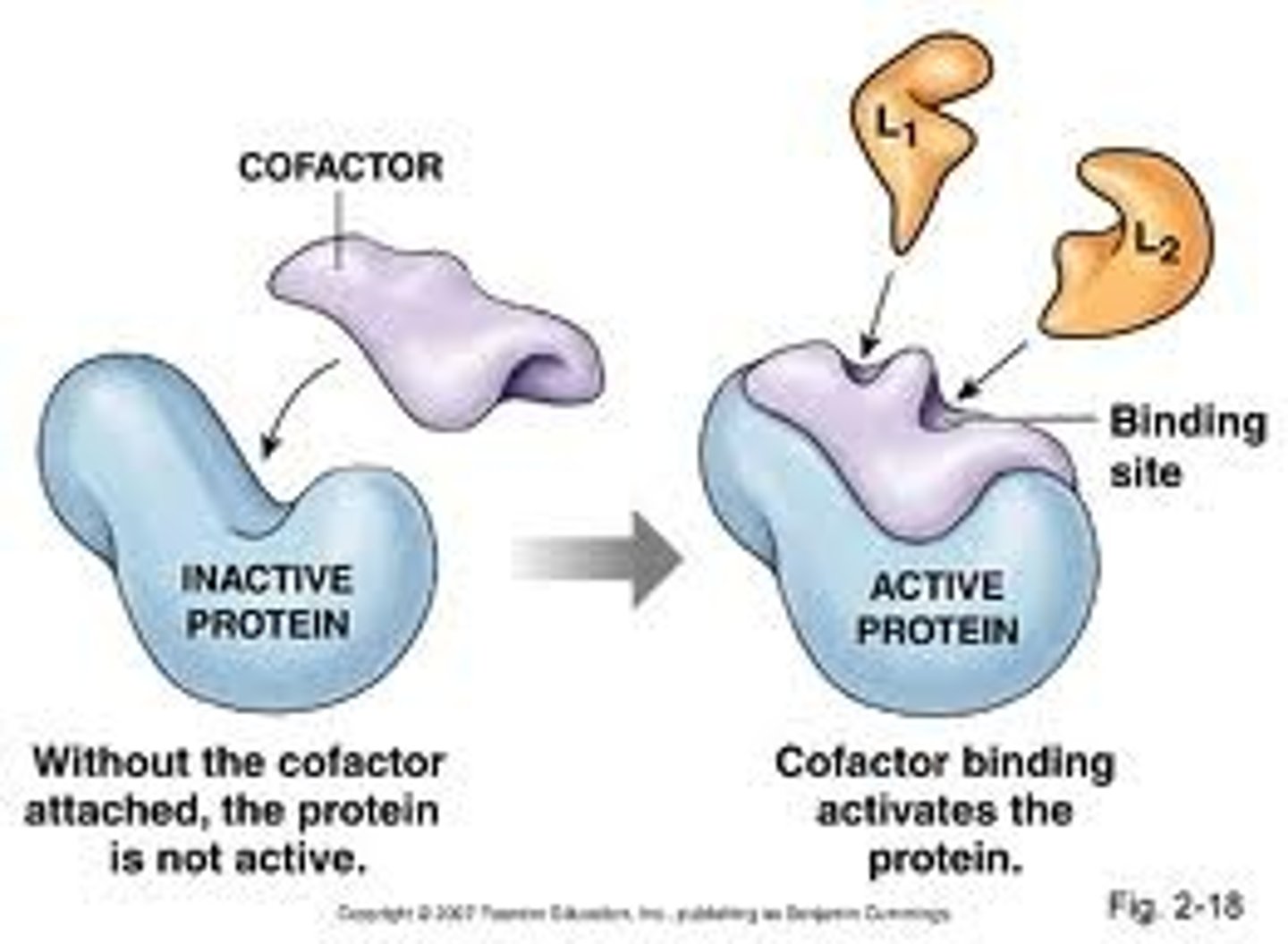
Cofactors
Inorganic, nonprotein helpers (ex. zinc, iron, copper)
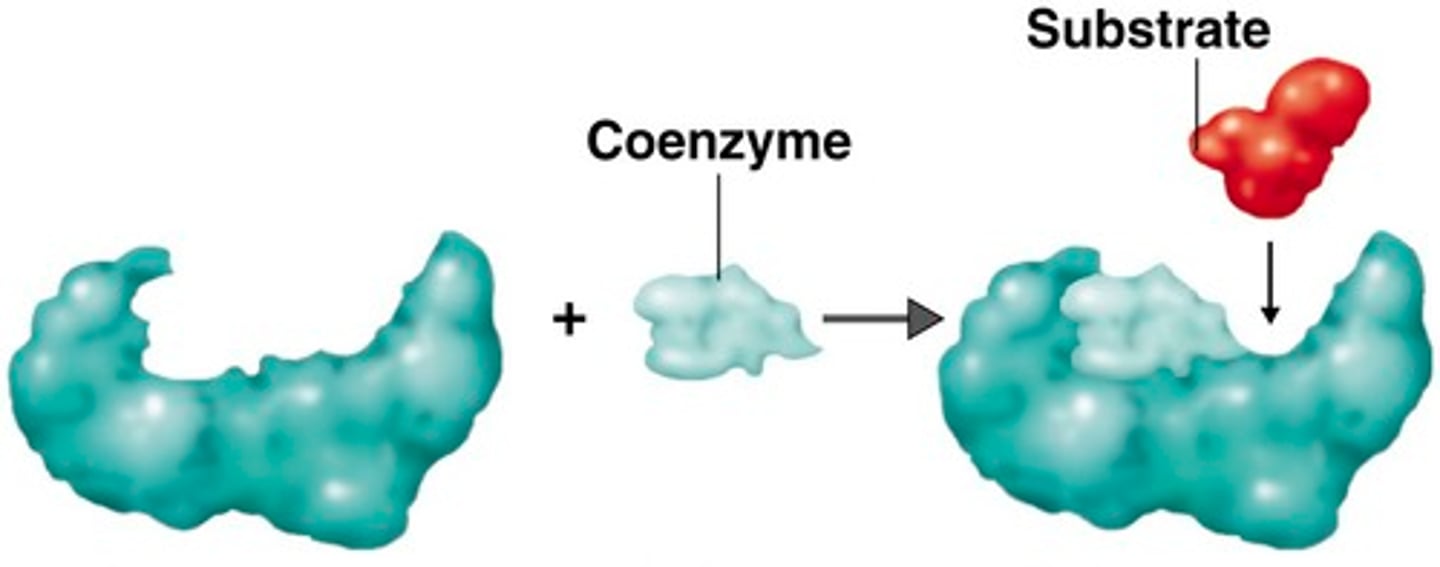
Coenzyme
Organic helpers (ex. vitamins)
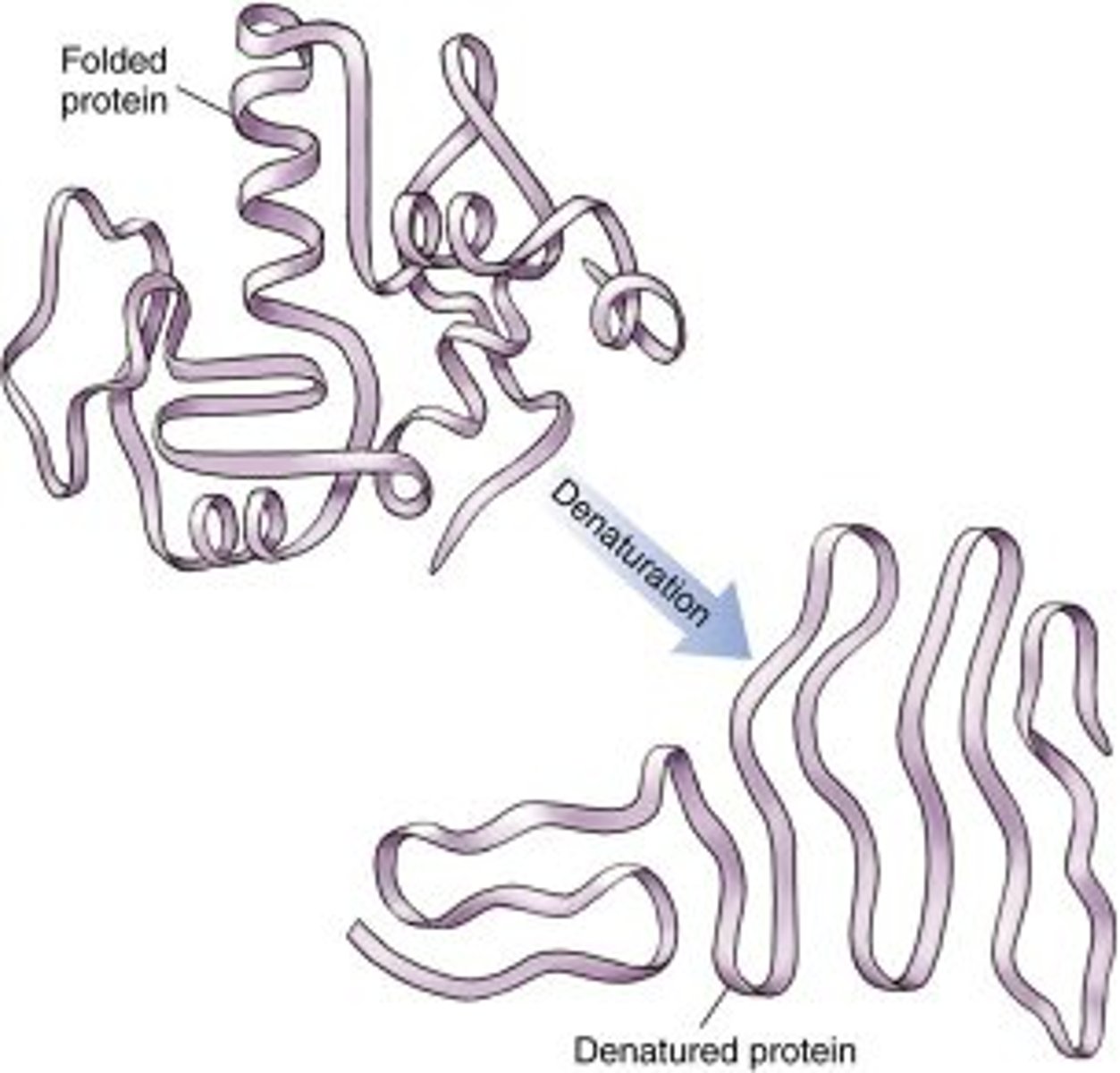
Denaturation
Structural change in a protein that results in the loss (usually permanent) of its biological properties
What is enzyme inhibition?
An inhibitor stops the substrate from reaching the active site
Competitive inhibition
Inhibitor competes for active site, mimics substrate (ex. Penicillin and enzyme for cell wall synthesis in bacteria)

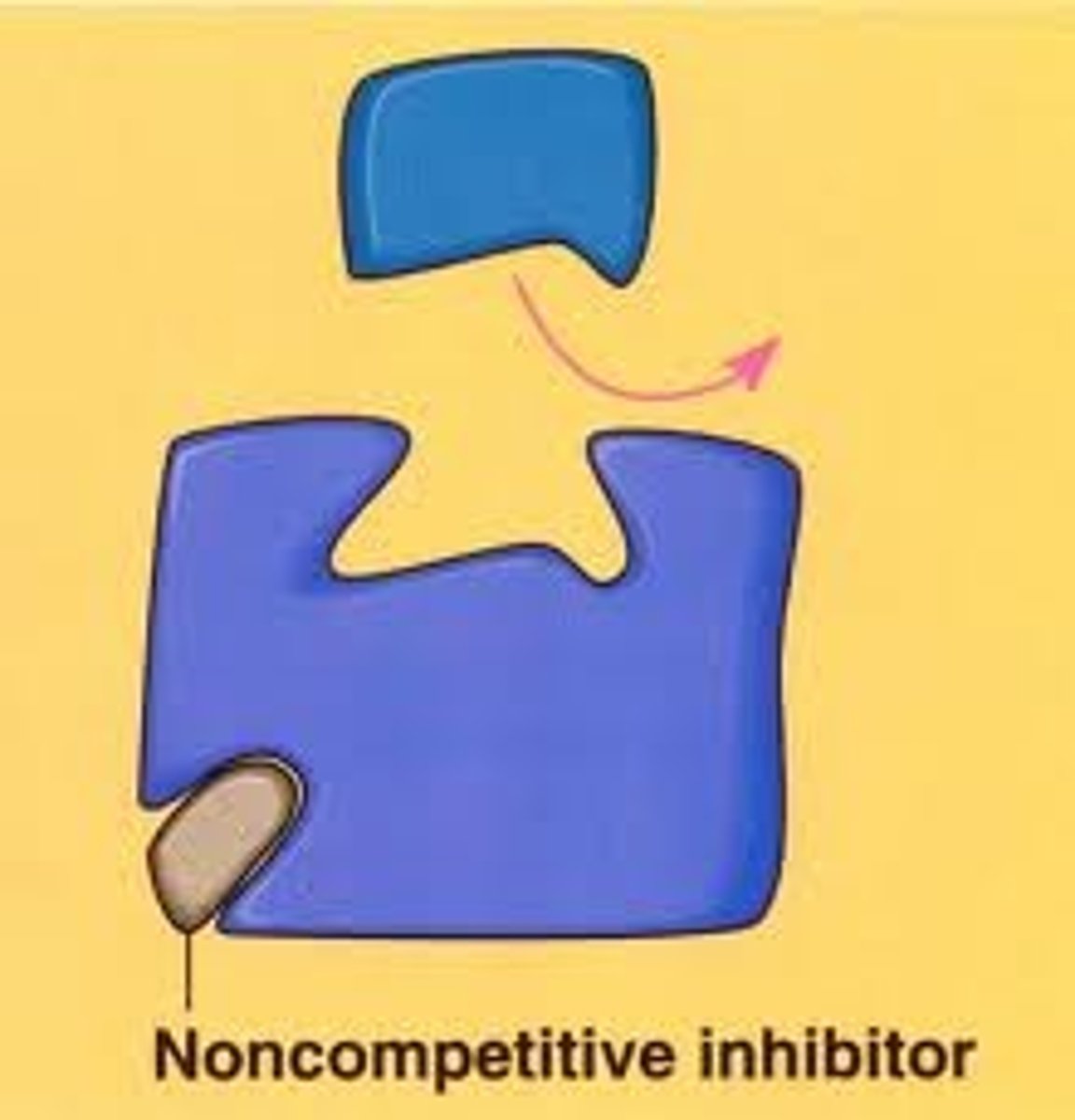
Noncompetitive inhibition
Inhibitor binds to another part of the enzyme (allosteric site) altering its conformation (shape); ex. heavy metal poisoning (heavy metals bind to allosteric sites on enzymes)
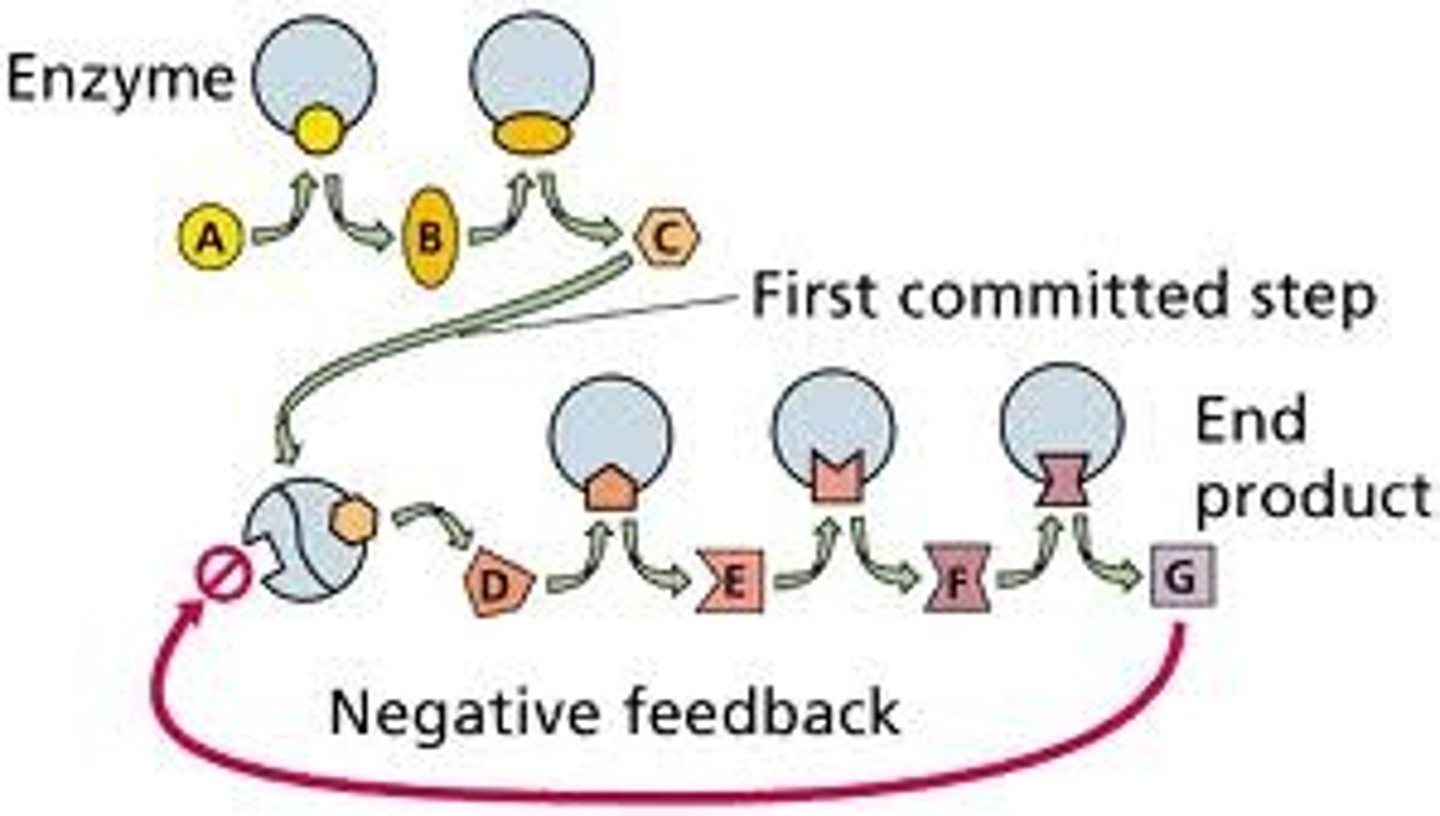
End product inhibition/Feedback inhibition
The end product of pathway acts as an inhibitor for an enzyme in the pathway, thus slowing it down.
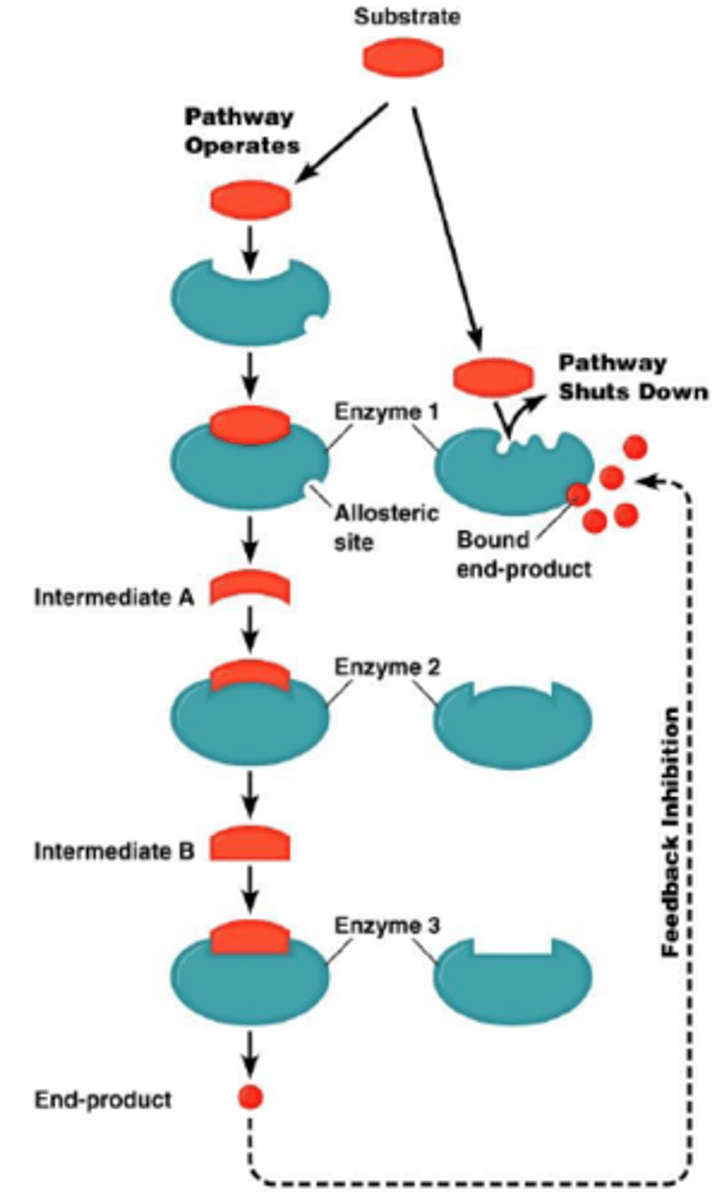
Explain the graph of the effects of inhibition on enzyme kinetics
Look at the top graph
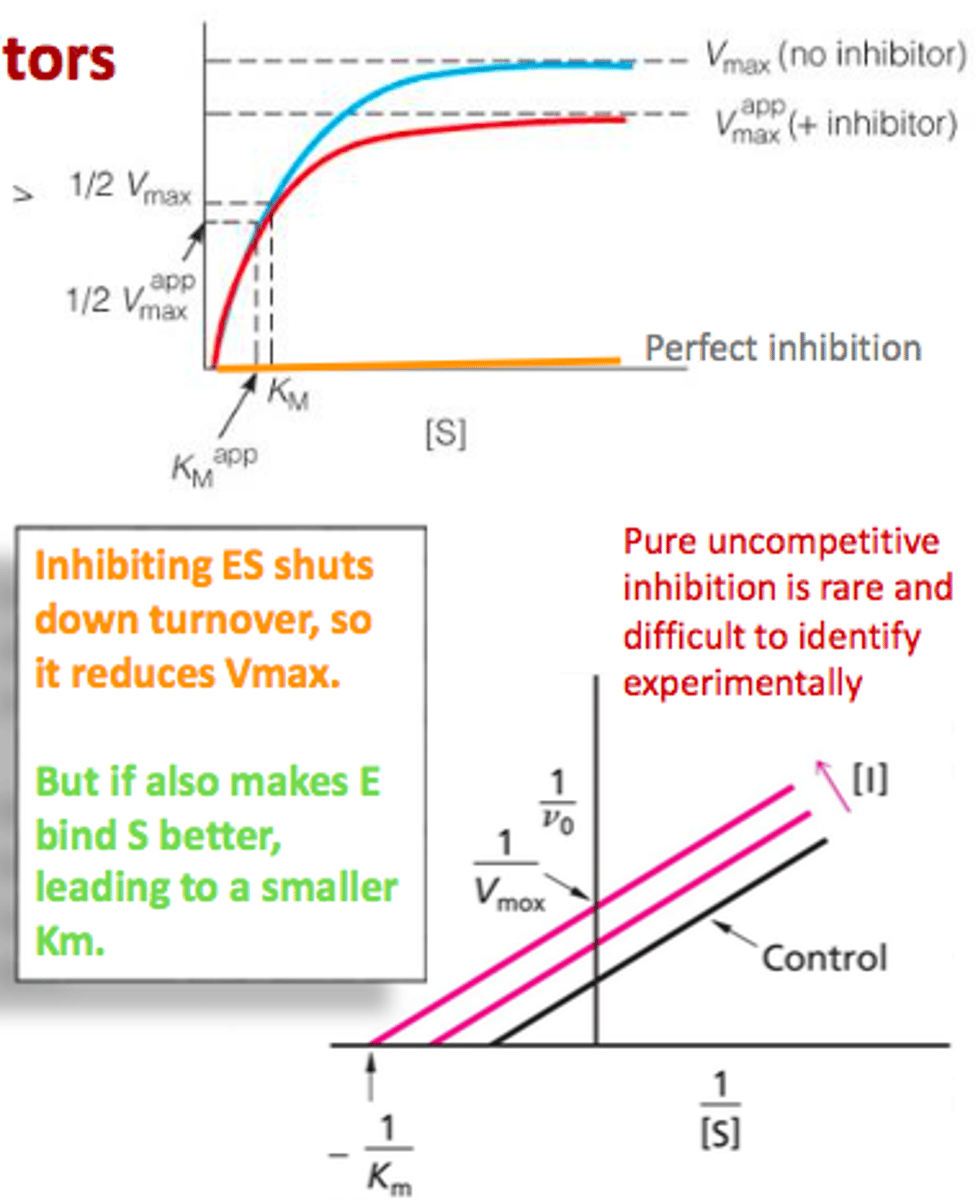
Give an example of enzyme inhibition in medicine
ex. Ethanol is used as competitive inhibitor for alcohol dehydrogenate to treat antifreeze poisoning
Give examples of protein utilization in industry
Proteins produced by cultured cells in fermenters are used in:
-foods
-pharmaceuticals
-other products (enzymes in cleaning agents etc.)

How immobilized enzymes are used in industry (some examples, as well)
-easy to separate from product
-can reuse enzymes
-can have higher enzyme concentrations
-increases enzyme stability
-ex. Lactase
Lactase obtained from cultured kluyveromyces lactis, a yeast that grows in milk
a) added to milk
b) immobilized on a surface or in beads of porous material (alginate beads)
