Chapter 3: Mass-Mole-Particle Relationships
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Mass
Amount of matter in grams.
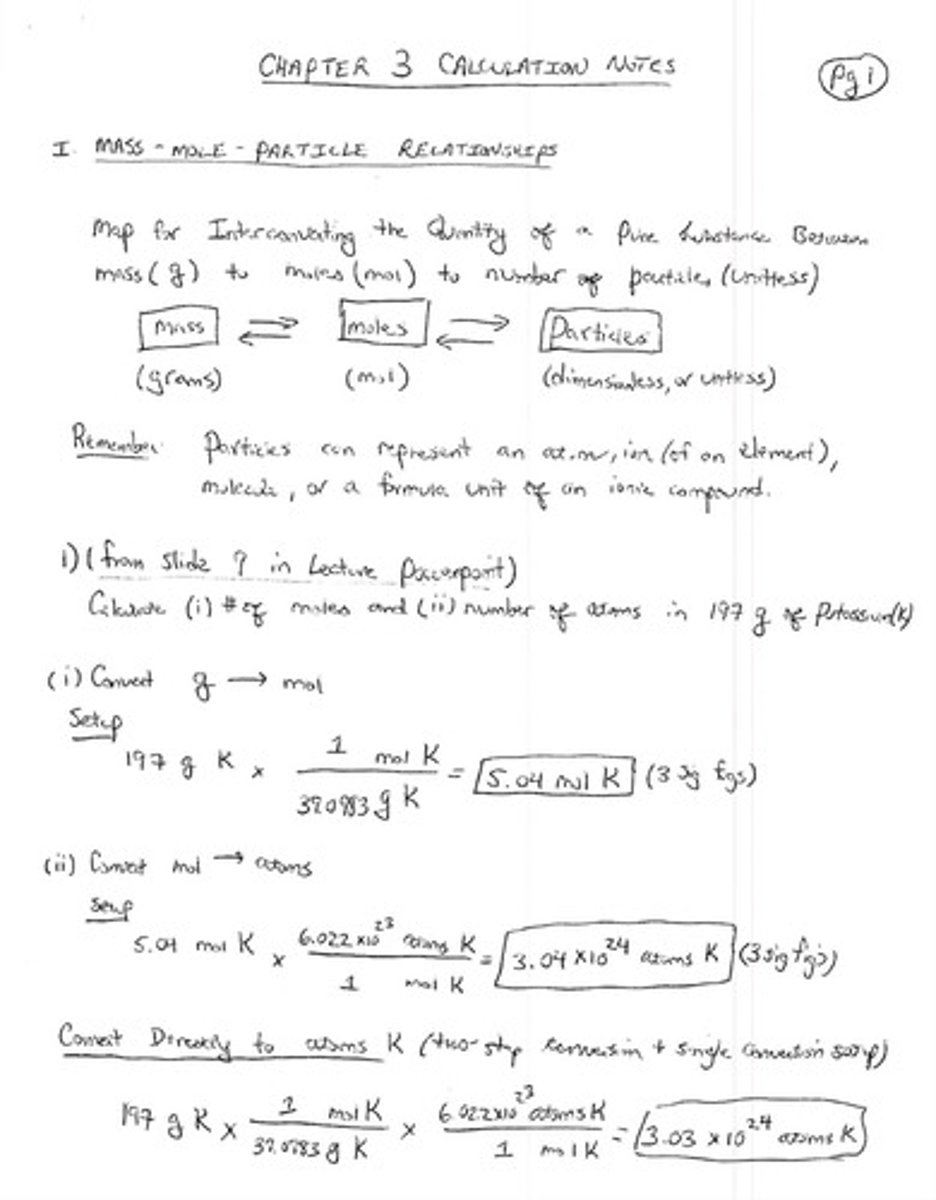
Mole
Unit for amount of substance, 6.022 x 10²³ particles.
Particle
Smallest unit of a substance, can be atom or molecule.
Conversion Factor
Ratio used to convert between units.
Molar Mass
Mass of one mole of a substance in grams.
Avogadro's Number
6.022 x 10²³, number of particles in a mole.
Stoichiometry
Calculation of reactants and products in chemical reactions.
Limiting Reactant
Reactant that is completely consumed in a reaction.

Excess Reactant
Reactant that remains after the reaction is complete.
Percent Composition
Percentage by mass of each element in a compound.

Empirical Formula
Simplest whole number ratio of elements in a compound.

Molecular Formula
Actual number of atoms of each element in a molecule.

Theoretical Yield
Maximum amount of product expected from a reaction.

Actual Yield
Amount of product actually obtained from a reaction.
Percent Yield
Actual yield divided by theoretical yield, times 100.
Glucose
C₆H₁₂O₆, a simple sugar used for energy.

Ammonium Sulfate
Chemical compound (NH₄)₂SO₄, used in fertilizers.

Benzene
C₆H₆, a known carcinogen and organic compound.

Iron (III) Carbonate
Fe₂(CO₃)₃, a compound with iron and carbonate ions.
Combustion Reaction
Chemical reaction where a substance combines with oxygen.
Haber-Bosch Process
Industrial method for synthesizing ammonia from nitrogen and hydrogen.
Chemical Equation
Symbolic representation of a chemical reaction.
Glucose Molar Mass
180.18 g/mol, mass of one mole of glucose.
Ammonium Sulfate Molar Mass
132.16 g/mol, mass of one mole of ammonium sulfate.
C4H10
Butane, a hydrocarbon used as fuel.
Molecule Count
Total number of molecules calculated from mass.
Mole Calculation
Conversion from grams to moles using molar mass.
Stoichiometric Coefficients
Numbers in front of compounds in a balanced equation.
Molecular Weight
Sum of atomic weights in a molecular formula.
Subscripts in Chemical Formula
Indicate the number of atoms of each element.