Protons, Neutrons, Electrons
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
location of protons
nucleus
charge of protons
positive
mass of protons
1 amu
location of neutrons
nucleus
charge of neutrons
neutral
mass of neutrons
1 amu
location of electrons
outside the nucleus, in the electron cloud/shell
charge of electrons
negative
mass of electrons
1/1870 - so negligible that we don't include it when calculating atomic mass)
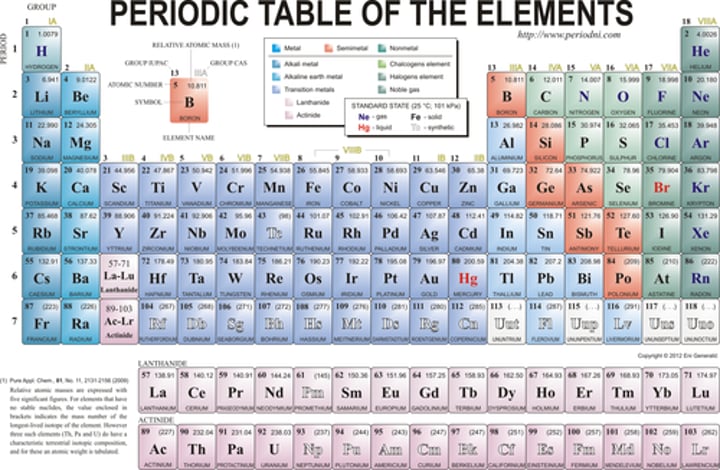
what is represented by atomic number
number of protons
Number of protons in an argon atom
18

Number of electrons in an argon atom
18

Number of neutrons in an argon atom
22

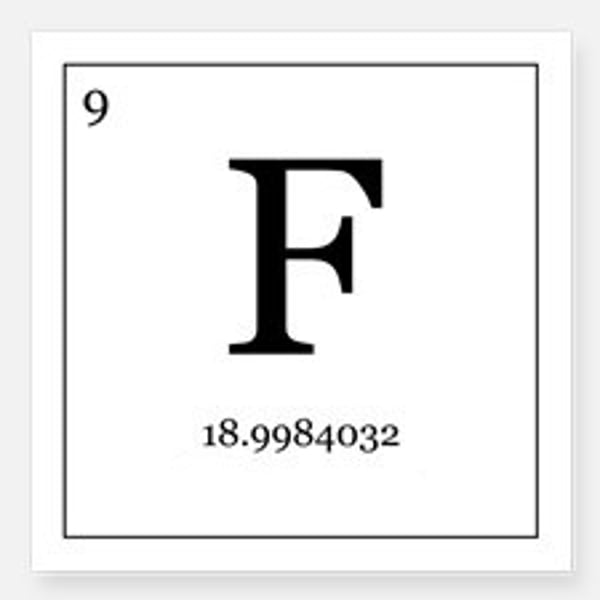
Number of protons in a fluorine atom
9
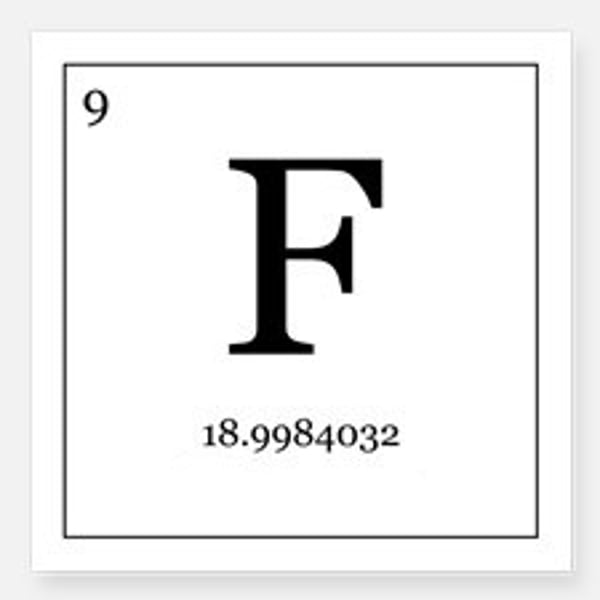
Number of neutrons in a fluorine atom
10
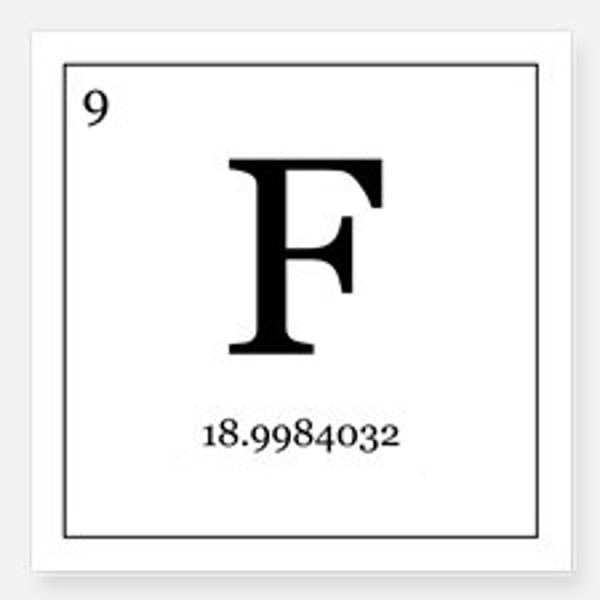
Number of electrons in a fluorine atom
9
Which element of the periodic table has an atomic number of 6
carbon
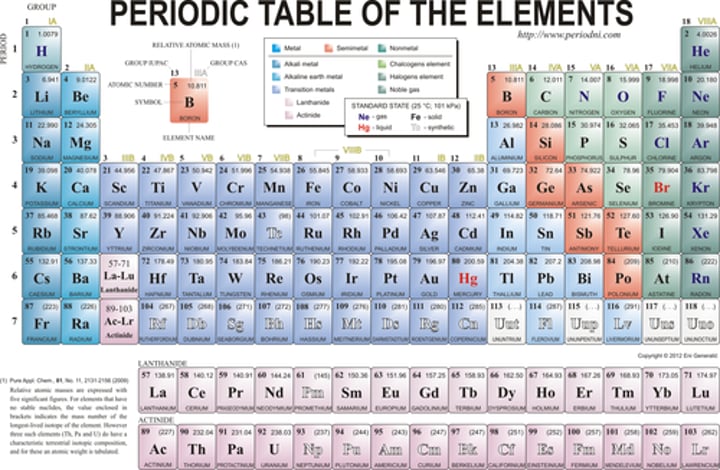
Which element of the periodic table contains 11 protons
sodium
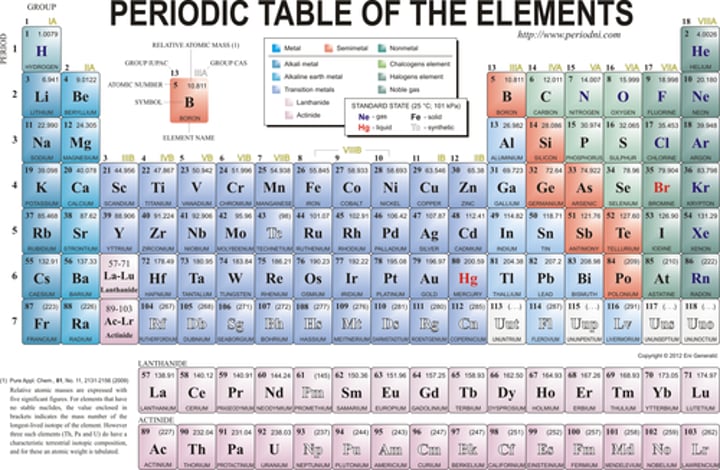
Which element of the periodic table contains 17 electrons when neutral
chlorine