energy changes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
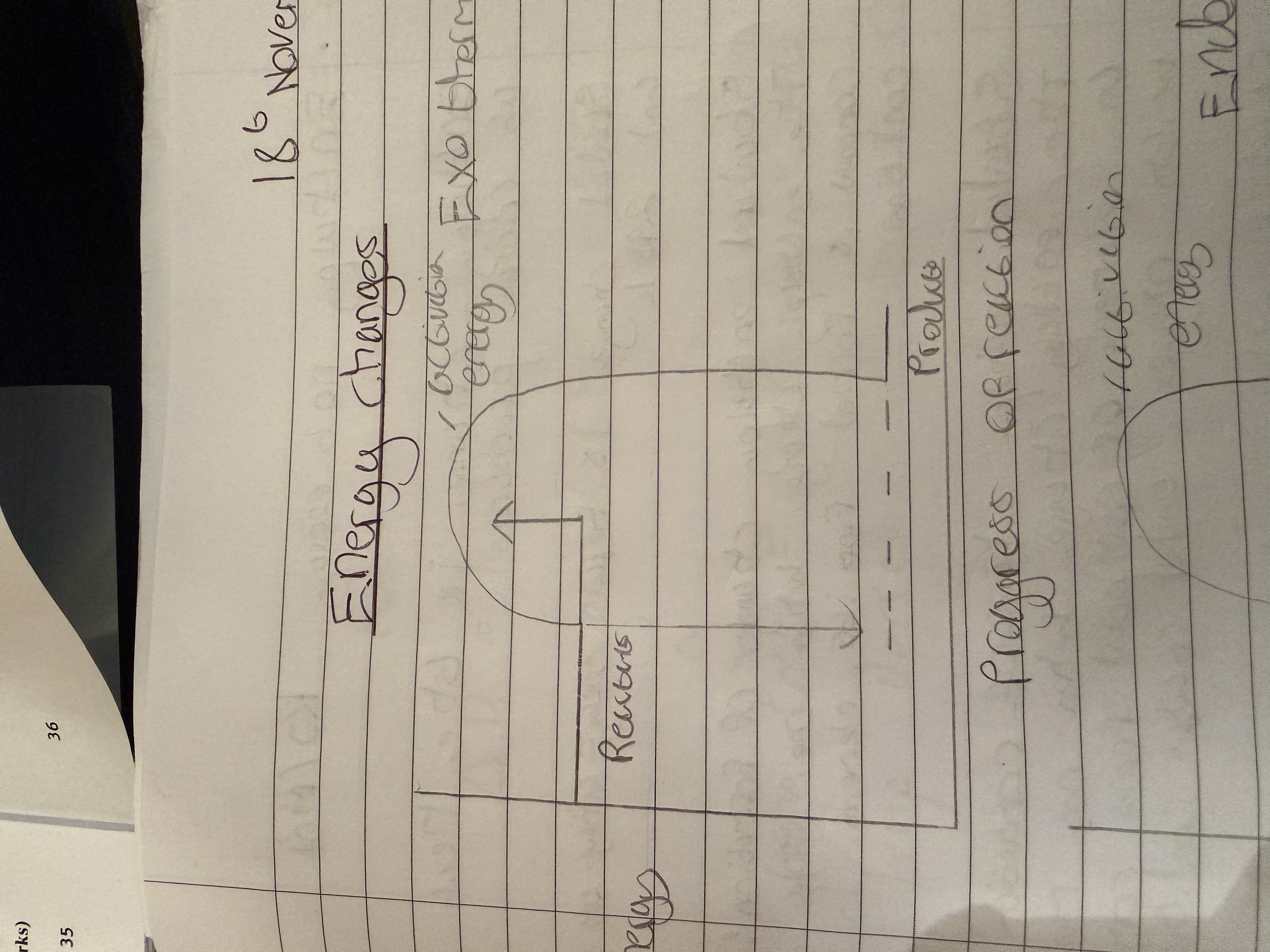
Exothermic reactions
Releases energy to the surroundings causing temperature to increase
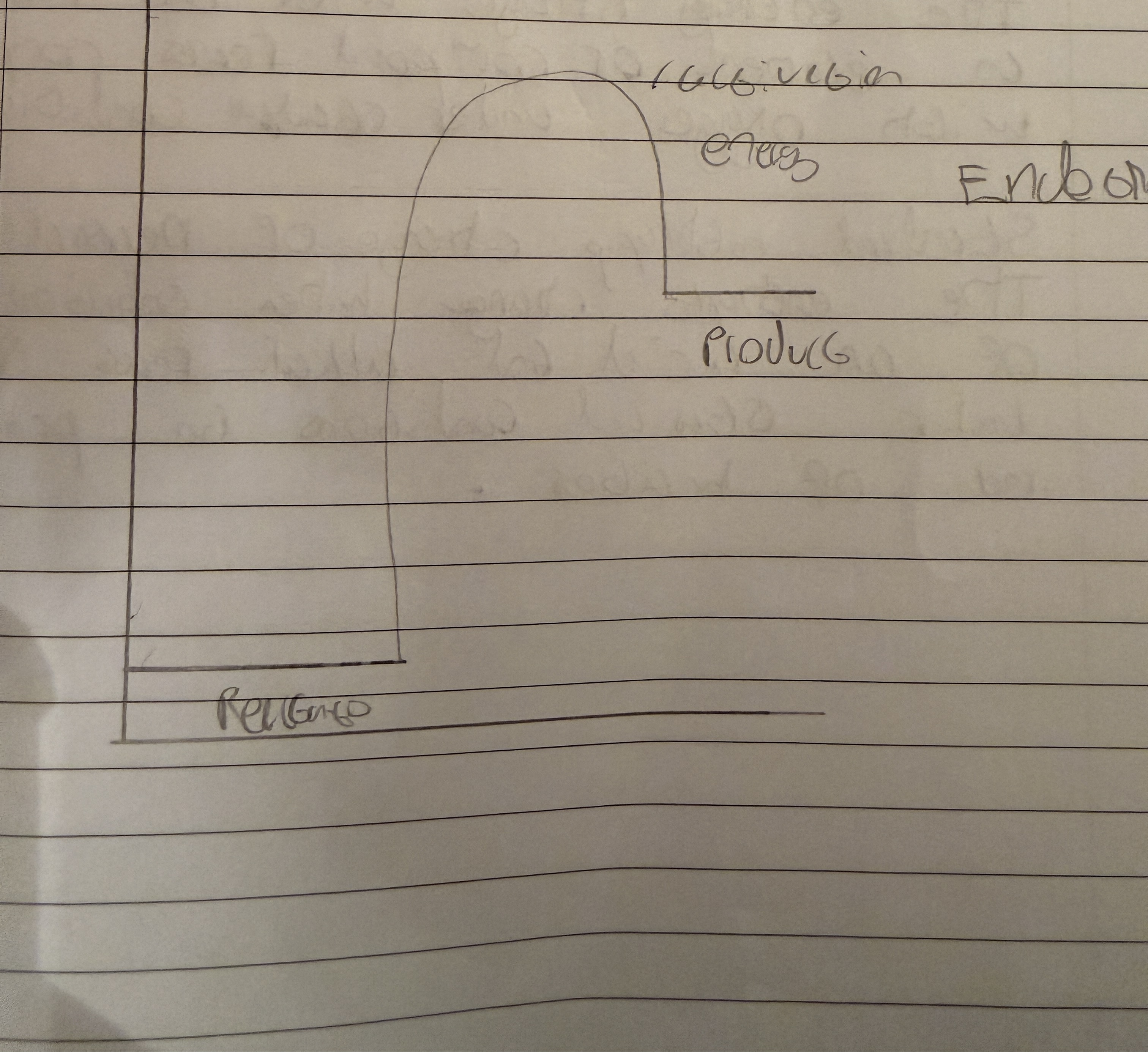
Endothermic reaction
Absorbs energy causing surroundings to cool
Enthalpy units
Kj/ mol
Enthalpy change definition
The heat change at constant pressure
Standard Enthalpy change definition
Heat energy change at 100kpa and 298k
Standard Enthalpy change of formation
Enthalpy change when one mole of a compound is formed from elements in standard conditions
Standard Enthalpy change of combustion
Then Enthalpy change when one mole of an element or compound reacts completely with oxygen under standard conditions
Standard Enthalpy change of neutralisation
Enthalpy change when an acid and alkali reacts completely together under standard conditions to produce one mole of water