Chapter 12- Lipids and Biological Membranes
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
Lipids
Water-insoluble biomolecules that are highly soluble in organic solvents
most lipids are hydrophobic due to fatty acids
Fatty acids
Long hydrocarbon chains that terminate with carboxylic acid groups
vary in length and degree of saturation
Often referred to in their carboxylate form because they are ionized at physiological pH
Fatty acid naming
Derived from the parent hydrocarbon by substitution of oic for the final e
First number is the number of carbon atoms in the chain, and the second number is the number of double bonds
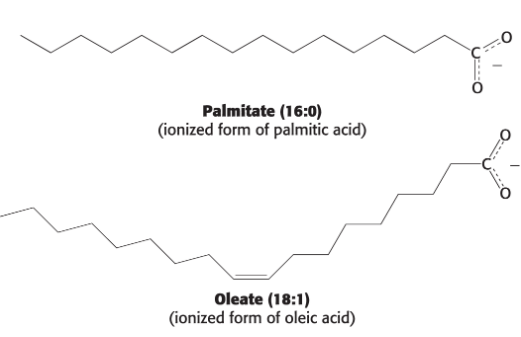
Fatty acid numbering can be done two ways:
Carbons can be numbered starting at the carboxyl terminal carbon atom.
Carbon atoms 2 and 3 are often referred to as α and β, respectively.
Position of a double bond can be represented by the symbol ∆ followed by a superscript number (examples:
cis-∆9, trans-∆2).The methyl carbon atom at the distal end of the chain is called the omega (ω) carbon.
Position of a double bond can be represented by counting from the distal end.
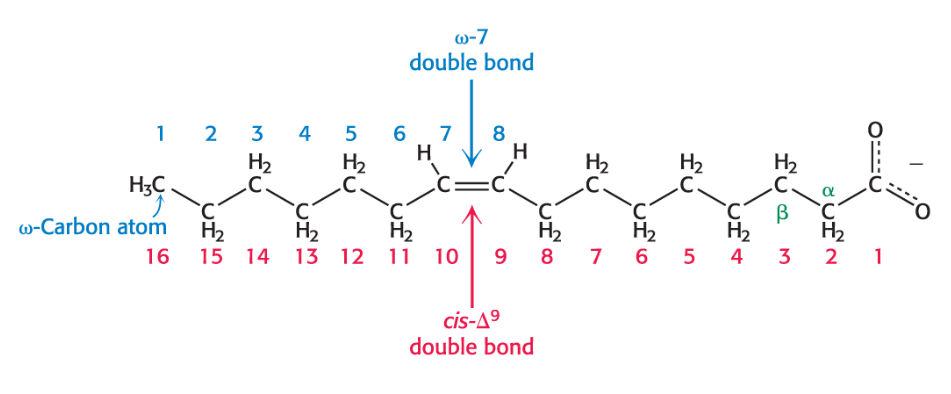
Chain Length and Degree of Saturation in fatty acid properties
Fatty acids in biological systems contain:
an even number of carbon atoms between 14 and 24 (16 and 18 are most common).
an unbranched hydrocarbon chain in animals.
a saturated or unsaturated (with double bonds in cis configuration) alkyl chain.
Short chain length and the unsaturation enhance the fluidity of fatty acids and their derivatives.
Unsaturated fatty acids have lower melting points than saturated fatty acids of the same length
shorter chain length = lower melting point
Lipids function as:
fuel molecules.
highly concentrated energy stores.
signal molecules and messengers in signal-transduction pathways.
the essential component of biological membranes
Principal lipids in eukaryotic membranes are
phospholipids, glycolipids, and cholesterol.
Common attributes of membranes
are sheetlike structures, two molecules thick, that form closed boundaries.
lipids, small molecules with hydrophobic and hydrophilic moieties that form lipid bilayers.
contain proteins embedded in lipid bilayers with distinct functions.
serve as pumps, channels, receptors, energy transducers, enzymes
are asymmetric, noncovalent assemblies.
outer and inner sections of the membrane are very different from each other
are fluid structures.
things can move around in the membrane
tend to be electrically polarized.
sum of charges on the inside of a cell is different than the sum of charges on the outside of a cell
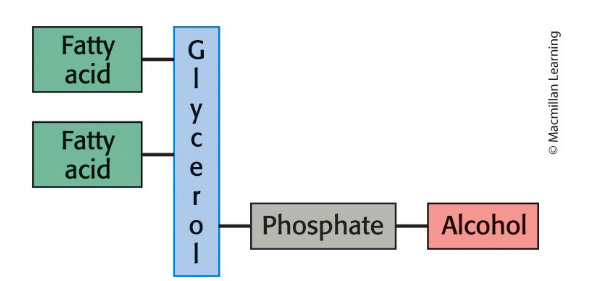
Phospholipid composition
one or more fatty acids.
provides a hydrophobic barrier
a platform to which the fatty acids are attached (examples: glycerol, sphingosine).
a phosphate.
an alcohol attached to the phosphate.
Sphingomyelin
Common membrane phospholipid with a sphingosine backbone instead of glycerol
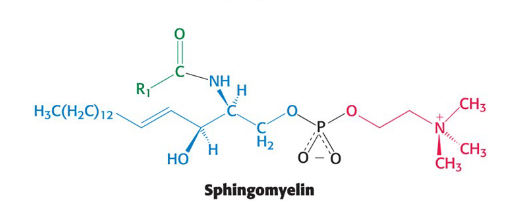
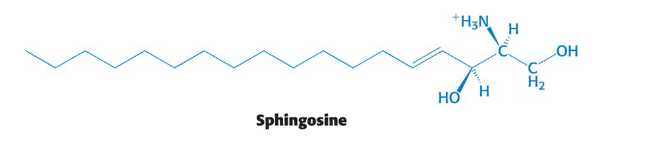
Sphingosine
an amino alcohol that contains a long, unsaturated hydrocarbon chain
Glycolipids
Lipids containing a sphingosine backbone with 1+ sugars attached to the primary -OH group
sugar residues are always on the extracellular side of the membrane
asymmetric
Cerebroside
Glycolipid containing a single glucose or galactose residue
Simplest glycolipid
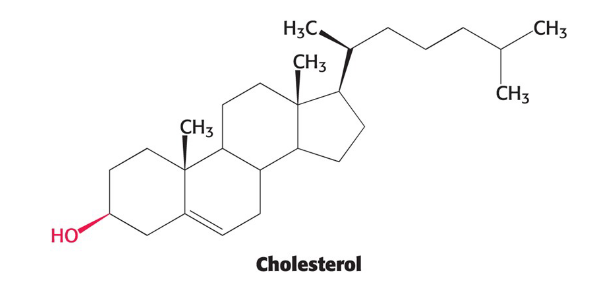
Cholesterol
Lipid based on a steroid nucleus
contains a linked hydrocarbon tail at one end and an -OH group at other end
Oriented parallel to fatty acid chains of phospholipid in membranes
-OH group interacts with phospholipid head groups
helps with fluidity of the membrane
Amphipathic (amphiphilic) molecules
Molecules that contain both a hydrophobic and hydrophilic moiety
Membrane lipids are amphipathic molecules
hydrophobic moiety: fatty acid tails
hydrophilic moiety: phosphorylcholine (polar head group)
How do phospholipids and glycoproteins form bimolecular sheets (membrane) in water?
Membrane formation is due to the amphipathic nature of the molecule
micelle will form
Micelle
A globular structure with the polar head groups on the outside surface and hydrocarbon tails sequestered inside
Lipid bilayers (bimolecular sheet)
Two lipid sheets
hydrophobic tails of each sheet interacting with one another, forming a permeability barrier
Hydrophilic head groups interact with the aqueous medium.
True or false: lipid bilayer formation is spontaneous
True
Phospholipids and glycolipids, because of the space taken up by their two tails, do not form small micelles the way single-tailed salts of fatty acids do.
Phospholipids and glycolipids spontaneously form lipid bilayers in aqueous solutions, stabilized by:
hydrophobic interactions.
van der Waals interactions between hydrocarbon tails.
electrostatic and hydrogen-bonding attractions between polar head groups and water molecules.
Langmuir-Blodgett trough
Are bimolecular sheet or micelle formation favored?
Bimolecular sheets
Biological Consequences of hydrophobic interactions
Lipid bilayers have an inherent tendency to be extensive.
Lipid bilayers will tend to close on themselves so that there are no edges with exposed hydrocarbon chains.
forms compartments
Lipid bilayers are self-sealing.
A hole in a bilayer is energetically unfavorable.
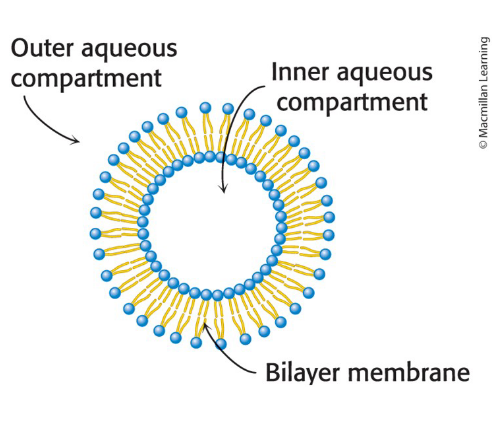
Lipid vesicles (liposomes)
Aqueous compartments enclosed by a lipid bilayer
used to study membrane permeability or to deliver chemicals to cells
Can be formed from phospholipids
Liposomes containing trapped ions/molecules can be synthesized
Formed by suspending a lipid in aqueous medium and sonicating (agitating by high frequency sound waves).
Ions or molecules can be trapped in the aqueous compartments by forming the vesicles in their presence.
Specific membrane proteins can be embedded by solubilizing the proteins in the presence of detergents and then adding them to the phospholipids
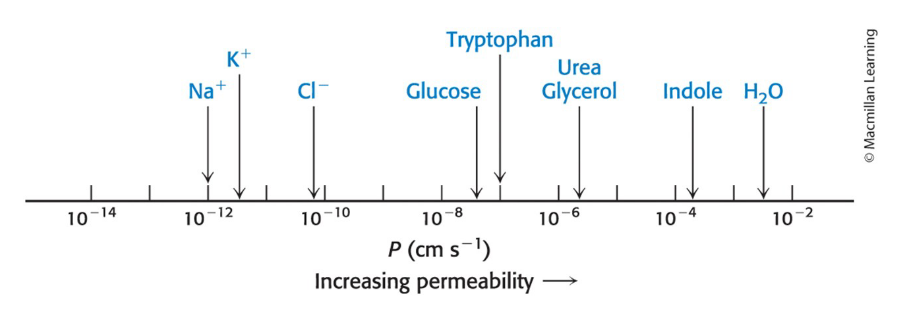
Lipid bilayer permeability
Lipid bilayer membranes have a very low permeability for ions and most polar molecules.
Permeability of small molecules is correlated with their solubility in a nonpolar solvent relative to their solubility in water.
Water is an exception due to its:
low molecular weight.
high concentration.
lack of complete charge.
Membrane proteins
Carry out most membrane processes
Allow transport of molecules and information across a membrane
establish compartments
Membranes vary in protein content
Ranges from <20% to as much as 75%
The types of membrane proteins in a cell are a reflection of the biochemistry occurring inside the cell
Can be visualized by SDS-polyacrylamide gel electrophoresis
shows that membranes performing different functions contain different types of proteins
Integral membrane proteins
interact extensively with the hydrocarbon chains of membrane lipids
released by agents that compete for these nonpolar
interactionsmost span the lipid bilayer
Peripheral membrane proteins
bound to membranes primarily by electrostatic and hydrogen-bond interactions with the head groups of lipids
disrupted by adding salts or by changing the pH
often bound to the surfaces of integral proteins
may be anchored to the lipid bilayer by a covalently attached hydrophobic chain
What are most common structural motifs in membrane proteins?
Membrane-spanning α helices
Bacteriorhodopsin = integral membrane protein & light-powered proton pump in archaea
contain predominantly nonpolar amino acids in contact with the hydrocarbon core of the membrane or with one another
polar and charged residues tend to be found in the cytoplasmic and extracellular regions
Beta strands
forms a channel protein
porin = protein from the outer membranes of bacteria
formed from a single anitparallel beta sheet curled up to form a pore or channel
outer surface is nonpolar
inner surface is hydrophilic and filled with water
contains alternating hydrophobic and hydrophilic amino acids along each Beta strand
Prostaglandin H2 synthase-1
ER membrane-bound enzyme that promotes inflammation and modulates gastric acid secretion
homodimer
lies along the outer surface of the membrane
bound by a set of α helices that extend from the bottom of the protein into the membrane
classified as an integral membrane protein even though it does not span the membrane because detergent is required to release the protein
linkage is strong enough that only the action of detergents can release the protein from the membrane
catalyzes the formation of prostaglandin H2
arachidonic acid = a hydrophobic molecule generated by the hydrolysis of membrane lipids
reached the Prostaglandin H2 Synthase-1 Active Site Through a Hydrophobic channel
Aspirin inhibits the cyclooxygenase activity of prostaglandin H2 Synthase-1
Aspirin inhibits cyclooxygenase activity by transferring its acetyl group to a Ser 530 in prostaglandin H2 synthase-1
Ser 530 lies along the path to the active site
Asprin blocks the channel
Lipid and many membrane protein diffusion
Diffuse rapidly in the plane of the membrane
Biological membranes are not rigid, static structures.
lateral diffusion = process by which lipids and many membrane proteins are constantly in lateral motion
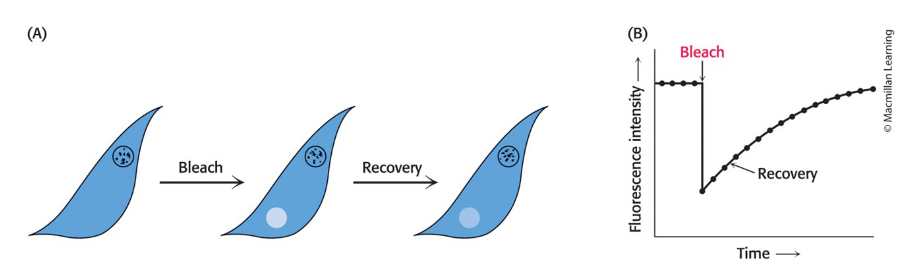
can be visualized for proteins via the technique of fluorescence recovery after photobleaching (FRAP)
Fluorescence Recovery After Photobleaching (FRAP)
A cell-surface component is labeled with a fluorescent chromophore and visualized via fluorescence microscopy.
Fluorescent molecules in a region are destroyed (bleached) by an intense light pulse from a laser.
Fluorescence is subsequently monitored as a function of time using a low light level.
prevents further bleaching
FRAP Recovery
a process resulting in an increase in the fluorescence intensity
occurs if the labeled component is mobile because bleached molecules leave and unbleached molecules enter
measures lateral diffusion
Rate of recovery depends on the lateral mobility of the fluorescence-labeled component.
Average distance S traversed in time t depends on a diffusion coefficient, D according to the expression S = (4Dt)1/2
Fluid mosaic model
Describes the biological membrane organization as 2-D solutions of oriented lipids and globular proteins
lipid bilayer is both a solvent and permeability barrier
Lipids rapidly diffuse laterally in membranes
Transverse diffusion (flip-flopping is very slow)
Proposed by Singer and Nicholson in 1972
Allows for lateral movement but not rotation through the membrane
Is lateral or transverse diffusion of lipids faster?
Lateral
Flip-flop (transverse diffusion) of a protein molecule has not been observed
preserves membrane asymmetry
What is membrane fluidity controlled by?
Fatty acid composition and cholesterol content
Many membrane processes depend on the fluidity of the membrane
depends on properties of fatty acid chains
the transition from rigid to fluid state takes place above the melting temperature (Tm)
As temperature increases, membrane changes from a packed ordered state to a more random one
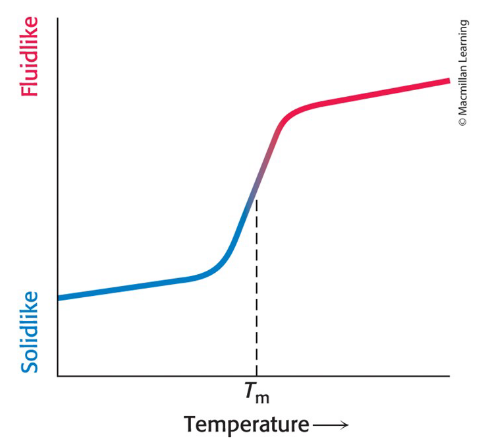
What does melting temperature of a fluid membrane depend on?
Degree of unsaturation
Straight hydrocarbon chains of saturated fatty acid residues interact favorably with one another.
favors the rigid state
A cis double bond produces a bend in the hydrocarbon
chain interferes with a highly ordered packing of fatty acid chains.lowers the Tm
Chain length
Long hydrocarbon chains interact more strongly than short ones
each additional CH2 group contributes about -2 kH mol to the free energy of interaction of two adjacent hydrocarbon chains
What disrupts the highly ordered packing of a fatty acid chain in a membrane?
Presence of cis double bonds
Cholesterol
the bulky steroid nulceus of cholesterol disrupts the regular interactions between fatty acid chains
helps maintain proper membrane fluidity in membranes in animals
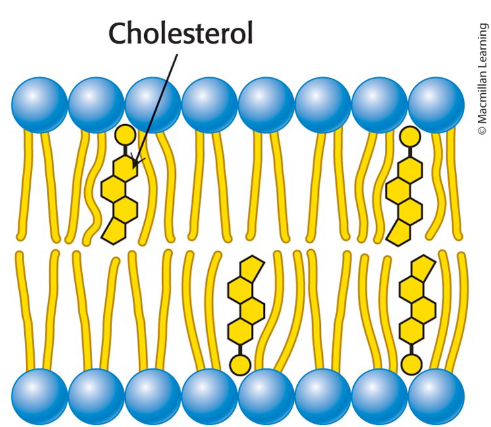
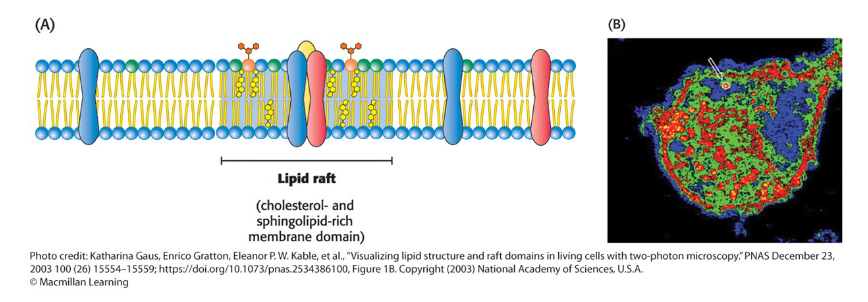
Lipid Rafts
Membrane domains containing such complexes that exhibit reduced fluidity
may induce conformational changes in membrane proteins to regulating their functional activities
may function in signal transduction by providing a
favorable environment for specific protein–protein
interactions
Highly dynamic complexes formed between cholesterol and specific lipids
Cholesterol can form complexes with lipids that contain the sphingosine backbone and with lipid-anchored membrane proteins
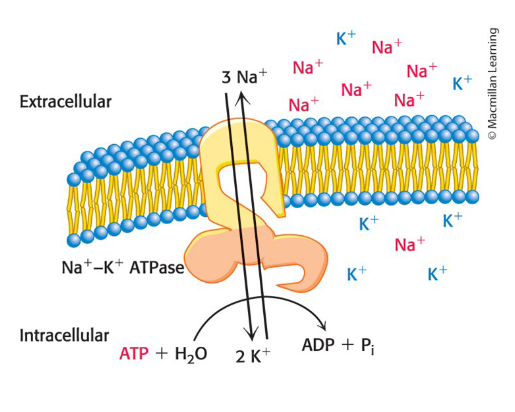
True or false: all biological membrane are asymmetric
True
The outer and inner surfaces of all biological membranes have different components and different enzymatic activities.
example: the Na+–K+ pump in the plasma membrane
ATP must be on the inside of the cell to drive the pump
What can bacteria be classified into based on their usage of biological membranes
Gram positive = have a single membrane surrounded by a thick cell wall
Gram negative = have two membranes separated by periplasm (which contains the cell wall)
Gram staining
Technique for classifying bacteria and distinguishing bacterial membranes
crystal violet strain (purple) is added to bacteria
iodine is added to trap the stain in the cell
alcohol is added to wash out the stain
a secondary stain (pink) is added to stain cells
Gram positive cells stain purple because their thick cell wall retains the crystal violet stain
Gram negative cells stain pink because their thin cell wall does not retain the crystal violet stain
Receptor-mediated endocytosis
process by which cells take up molecules from their environment
Receptor binding induces membrane invagination by the action of intracellular clathrin and dynamin.
membrane budding and fusion are highly controlled processes
Vesicle fusion to the plasma membrane is critical for neurotransmitter release from a neuron into the synaptic cleft
RME of transferrin receptor mediates cellular uptake of iron
Free iron is toxic to cells and tightly bound to transferrin in the bloodstream.
Complex formation between the transferrin receptor and iron-bound transferrin initiates receptor-mediated endocytosis.
leads to internalization of these these complexes within vesicles (endosomes)
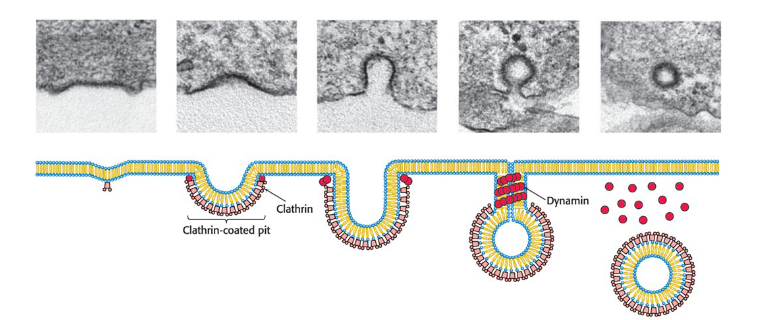
SNARE Complexes
Guide membrane fusion to ensure specificity
Draw appropriate lipid bilayers together through the formation of tightly coiled four-helical bundles
largely determine the compartment with which a vesicle will fuse
Initiate membrane fusion