Lecture 16 Adsorbed Proteins
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
=
Proteins hierarchical structure
Primary structure
Secondary structure
Tertiary structure
Quaternary structure
Protein dynamics
Protein bonds are vibrating, twisting, reorienting
molecules
amino acid side chains
Amino acid side chain
Charged (acidic/basic)
Non-charged, polar
Non-charged, non-polar
Define relevant biological components
Cell cytoskeleton
Cellular membrane
Integrin
Extracellular matrix
Tissue
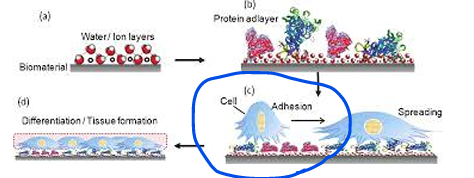
List in chronological order the biological component interaction with biomaterials
water
proteins
cells
Identify properties that influence protein adsorption
Implanting artificial material vs donor tissues (allografts)
Protein adsorption may alter the material interface
Study protein adhesion on specific materials and the resulting cell attachment to determine biocompatibility
Rapid Adsorption Kinetics and Irreversibility
Study protein adhesion on specific materials and the resulting cell attachment to determine biocompatibility
Protein adsorption may alter the material interface
Polystyrene cell culture dish
Expose to protein solution, water no longer beads up
Rapid Adsorption Kinetics and Irreversibility
Proteins initially adsorb quickly
More difficult over time for proteins to find and fit onto
surface
Monolayer of proteins
Competition in protein adsorption
Competition in protein adsorption
Specific proteins can have varying affinities for different
materials
Affinity for specific surface chemistry and mass
concentration in bulk phase determine competition of
protein adsorption
Vroman effect
Protein adsorption is transient
Molecular spreading events: conformational and biological changes in adsorbed proteins
Molecular spreading events
: conformational and biological changes in adsorbed proteins
"Soft" proteins may change shape and are more adsorptive
"Hard" proteins are more stable in bioactivity
"Soft" proteins may
change shape and are more adsorptive
"Hard" proteins are
more stable in bioactivity
Study protein adhesion on specific materials and the resulting cell attachment to determine biocompatibility
Typically study with in vitro experiments
Typically study with in vitro experiments
Single protein
Multi-protein
Single protein in a
buffer solution
Multi-protein solution like
blood plasma
tissue integration means
control:
-water -(small high concentrations) (1st arrives) (surface characterization)
-protein-> major research area in vitro testing
-cells
-tissue-> in vivo
how they all integrate within the tissue
Protein
chain of amino acids
Protein primary structure
- peptide bonds, covalent bonds
-strong sequence of amino acids
-sets stage for secondary structure
protein secondary structure
- common interactions between carboxyl group + amine group of different amino acids (create the alpha helix or beta pleated sheet) are weak bonds can break apart spotted in tertiary stcture
so like 20% beta sheet and 8--% alpha or any other ratio
p
protein tertiary structure
- 3D shape of max stability and lowest energy
protein dynamic change shape in heat and interact with diff things
weak interactions between amino acids so like van der waals forces and hydrogen bonding
protein quaternary structure
- subunits many multichains, peptide
interaction between carboxyl and amine grupsthat cause interactions withing the beta sheet leads to formations that are alpha or beta get interactionsbetween amino acids between carboxyl and amine
Describe protein structure and dynamics
bonds twist and reoriant covalent dont break
but common for all structures to break and be lost
3rd and 4th postive chanrge and negative charge polar
amino acids only thing different is the
R group
can be nonpolar-methyl groups or hyrogens no charge
can be postive charge- NH2+ or carboxyls
polar groups-OH and amine groups Nh-
3d shape of protein is dependent on
amino acid interactions /behavior
nonpolar (hydrophobic) amino acids would be in the
center
of the protein
since interact less with environment
gives shape of the protein
-so where these are located in peptide chain would create shape since they all like to hang out in the center to avoid water
polar postive charge and negative charge all
outside or near outside
Cell cytoskeleton
formed by protein builds shape of cell structural support rebar of cells long structures proteins
long,strong proteins
dont need to know the specific
Cellular membrane
envelope of the cell, provides protection
have proteins in the cell membrane only certain things cana pass like water but cant pass can pass through channels made by proteins
Integrins
protein in the cell membrane and passes thorugh the cell membrane where it attaches the cell cytoskeleton to the tissue cytoskeleton which is called the extracellula matrix
(ECM)(- portein and tossu that provide support to the tissue))
is key to good cell integration
Extracellular matrix
integrims attach to this
Tissue
want integration
are cells an ECM (tissue)
-not just cells since strength comes from ECM and cells are not structural or strong the strength of bonesand stretchiness is because of ECM
cells and ECM found in each tissue is different
-implant medical device
-injury
-> initiates a wound healing response (can lead to tissue integration if go well if goes badly can lead to foreign body response)
-
as we cause injury we are
crushing capillaries
cutting vasculature
blood would come to the
injured area
blood-plasma (water ions and proteins)
can cause a clot made with fibrinogen (protein causes blood clotting in plamsma)
-
-serum=plasma-fibrinogen (serum is easy to work with to avoid clots from forming)
plasma and serum are both water+ios and proteins
separated by
density
bottom red blood cells (oxygen)
buffy area with white blood cells (platelets)
plasma(water+ions+proteins(salts an enzymes) all dissolved
is high concentration comes first and proteins are dependent on
water since hydriphobic vs hydrophilic
Water
(low molecular weight) diffuse fastest, reach biomaterial
first
Proteins
(from blood serum)
come to the materials and start interacting with the material then they spead
often proteins change shape with adsoption (adsorption-weak interactions between the protein and material surface)(more likely to happen with hydrophibic since distrupting the order )
-proteins spread out and increase attachment points between protein and material
shape of binding sites is key
cells
attach to certain proteins ex immune and white blood cells issue not tissue creates immune repsonses
like to attach are host tissue cells cells belonging to that tissue know to create ECM for that tissue
from blood or tissue area and attach to our adsorbed proteins via integrins then when attach spreads out and forms ECM then tissue differentiation (DNA->RNA->protein which happens to be ECM)
order of biological components
plasma (top)
WBC
RBC (bottom)
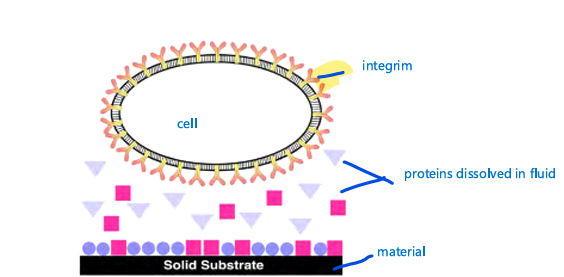
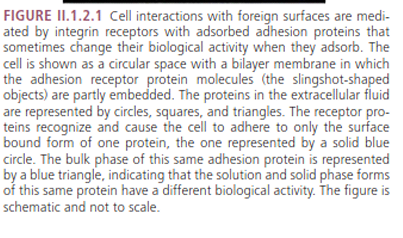
cells
cell would love to bind to
diff proteins and all around are integrins and integrins have specific binding site shape and in that binding site are specific amino acids so specific amino acids might have some positive or negative charges and provide selectivity on which protein to bind to
diff cells diff integrin types like skin or bone diff since binding to proteins in that are
once the protein adsorbs into the surface can change
shape (the protein may loose its shape of the binding site) so the triangle changes into a square
if the protein loses its shape it can change its
bioacitvity therefore where it binds to and can open new binding sites or remove binding sites
on a surface if hydrophobic particles are likely to interact with one another and adsorb and can become a binding site for integrins and once get absorbed can change shape so binding
protein configuration is
dynamic
binding site shape may change
when get adsorption can lose bioactivity but can also gain bioactivity what lost it can gain it and reverse so
bioactivity changes
when implant material is coded with proteins some might
change shape some might not get a monolayer of proteins adbsorbed onto a surface
if cell comes up on material, it recognizes the protein on it not the
type of material since it is coated with proteins
cells interact with the
protein layer'
so its which porteins track and attach diff cells onit
would like to have host tissue cells
- bone cells or skin cells or muscle cells (whtever cell is supposed to be in region it is being implanted into) its bad if attract and attach white blood cells, red blood cells, platlets
important protein layer
can we preadadsorb proteins?
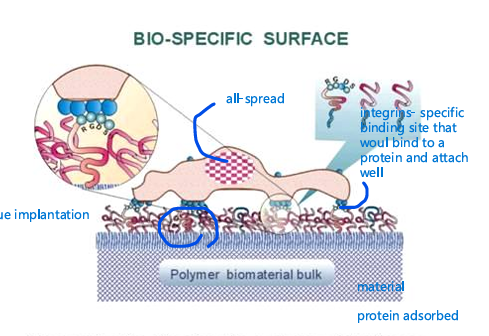
integrins
- specific binding site that would bind to a protein and attach well
integrins bind to
3 specic amino acids (RGD)
in a ton of proteins and bind to different intgrins
problem is that RGD is
super common in many proteins and
binds to many different integrins also
binds to platelets and cause blood
cloths as well
does not combine specifity needed
(RGD is promiscus amino acid sequence)
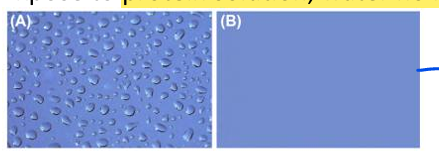
Protein adsorption may alter the material interface
Polystyrene cell culture dish
Expose to protein solution, water no longer beads up
Polystyrene cell culture dish
- water beads up (high contact
angle)
if have protein layer can change
hydrophobic or philic property
Expose to protein solution, water no longer beads up
protein adsorption leads it to be hydrophilic so protein layers can change behavior of surface
protein adsorption leads it to be hydrophilic so protein layers can change behavior of surface
polystyrene cell culture dish (if put water beads up but if expose to blood serum in cell culture water spreads out
Rapid Adsorption Kinetics and Irreversibility
Proteins initially adsorb quickly
More difficult over time for proteins to find and fit onto
surface
Monolayer of proteins
Competition in protein adsorption
Competition in protein adsorption
Specific proteins can have varying affinities for different
materials
Affinity for specific surface chemistry and mass
concentration in bulk phase determine competition of
protein adsorption
Vroman effect
Protein adsorption is transient
have material first interact is
water then small MW then high concentration proteins
one of the most common small me and high conc proteins is
albumin and there are blood serum proteins (proteins dissolved in blood
is most common out of all main job is control osmolarity in and outside the cell keep blood cell isotomic and is filler doesnt bind or do anything hangs around onmaterial surface but does nothing does not help in cell intergration) only has one or two points of connecting
3rd is getting larger MW and lower concentration proteins- bigger proteind
less in blood serum like fibernectin or fibernogen big proteins that have alot of points of contact with material (higher MW more liely to have more interaction points ad more likely to stay addsorbed)
these are weak interactions like vander waals forces and often break so chances of albumin readsorbing is high because there is only one or two points while te larger has more interactions that allow it to break and reform thus staying over for the long term
because of diffusion (smaller and high cocnentration meets material first vs other combo that we get this order but over thwe we have Varman effect (protein absorption dynamics larger would replace smaller and stay longer because more inreraction points)
protein adsopbrtion is transient so can
get adsorbed but also if "soft" too since it has weaker teritary,secondary and quernaty structure since it can unfold onto surgace and stay adsprbed while a rigid has more covalent bonds and more likely to stay in place
so protein more likely
bind to cells
soft spread out more interaction
but binding site integrations change
wont bind to a cell
this is also looked for in enzymes
"hard"-
proteins more likely to leave surface but also more likey to maintain bioactivity
example protein and binding site
available one adsorbed on biomaterial surface and might open a cryptic binding site that was not open before but now is
study by looking at single proteins in vitro
-adsorption rates
-conformational changes in proteins
-multi protein solutions so coat material with serum or plasma and then see protein competition
ex on surface would like to have fibranectin (protein known for good cell/bone attachment) we do not want fibrinogen which leads to platelet attachment causing blood cloths (bad!)''']
both have RGD on them but these control cell integrim bindings
protein adsorption
controls good and bad outcomes and biocompatibility
protein can either be
stable (hard) or unstable (soft) effect electrical charge and adsorption
add functional groups to surface as well
Cell cytoskeleton
is a fundamental, structurally highly organized, and functionally integrated assembly of proteins within the cell.
It forms the major contractile structure in most cells and plays a critical role in cellular mechanical functions, adhesion, and signaling
Cell membrane
critical boundary that defines the cell, mediates interactions with the environment, and is central to several biological processes, including mechanotransduction, molecular transport, and immunity.
Integrins
are a major class of transmembrane adhesion receptors that play a pivotal and indispensable role in mediating cell-Extracellular Matrix (ECM) interactions and regulating critical cellular functions
link the ECM to the cytoskeleton (specifically actin filaments) through focal adhesion complexes. This linkage transmits mechanical forces from the ECM to the nucleus, which can alter chromatin structure and regulate gene expression
form a mechanical linkage between the ECM (via adhesion sites called focal adhesions) and the internal cytoskeleton (actin filaments). They regulate vital cellular functions, including cytoskeletal organization, proliferation, differentiation, apoptosis, and migration
Extracellular Membrane (ECM)
vital, complex, and dynamic biological material that serves as the microenvironmental niche in which cells reside, adhere, and function within tissues and organs. It is far more than just a passive scaffold; it actively regulates cell behavior and tissue integrity through a continuous exchange of physical and biochemical cues
proteins mediate cell adhesion, including Fibronectin (presenting the crucial RGD sequence) and Laminins (a major constituent of the basement membrane
Tissue
scaffolds are designed to serve as an artificial Extracellular Matrix (ECM) to restore, replace, or regenerate tissues lost due to disease or injures
Allografts
biological tissues or fragments obtained from a human donor or cadaver and transplanted into a recipient patient. They are widely used in medicine, particularly for replacement therapies where autologous (patient-derived) tissue is unavailable or unsuitable, or where synthetic materials fall short
Bulk proteins
the vast array of macromolecules present in biological fluids (like blood plasma and serum) that are critical reactants in the host response to implanted biomaterials.
Even if a protein has a high intrinsic affinity for a surface, it will not be present in high amounts on the surface if it is
present in low bulk concentration compared to other competing proteins.
For example, albumin, despite being a lower affinity protein than fibrinogen, is adsorbed to the surface in similar amounts because its bulk concentration is much higher, driving it onto the surface according to the law of mass action.
Adsorbed proteins
are a layer of biomolecules that rapidly and tenaciously form on the surface of any material exposed to a biological fluid (such as blood plasma or serum), and they are the primary determinant of the subsequent biological response to an implanted synthetic material
converts an inert, synthetic surface into a biologically recognizable material
are irreversibly bound to the surface; washing the surface with buffer does not remove them. This tight binding is due to the large size of the protein molecules, resulting in multipoint attachment where many weak, noncovalent bonds (like van der Waals, electrostatic, and hydrophobic interactions) form simultaneously between the surface and the protein, making the probability of all bonds breaking at once very low
The Vroman Effect
: This is an unusual competitive phenomenon where a protein (most notably fibrinogen) is initially adsorbed at high levels but is subsequently displaced by later arriving, more surface-active plasma proteins (such as high-molecular-weight kininogen).
is thermodynamically driven to lower the overall free-energy state of the system
Blood serum
(often referred to interchangeably with plasma in general physicochemical property discussions)
is the fluid component of blood defined by the absence of fibrinogen, a key coagulation protein
Blood plasma
is the aqueous medium component of blood that contains a complex mixture of proteins, ions, and organic compounds.
It is crucial in blood-material interactions (BMI) because it is the fluid phase from which proteins adsorb onto foreign surfaces, initiating coagulation and other host responses
is defined as the fluid component remaining after the blood has clotted, meaning it is plasma without fibrinogen
Due to the absence of fibrinogen (a large protein), the viscosity of serum is slightly less than that of plasma
Soft proteins
refers to proteins characterized by low thermodynamic stability.
These proteins exhibit specific behavior when interacting with surfaces:
• Adsorption: Soft proteins tend to adsorb more readily and more tenaciously than "hard" proteins, which are more stable.
• Conformation: Due to their low stability, soft proteins are prone to unfolding or conformational changes upon adsorption. These changes can be so profound that some soft proteins (e.g., lactalbumin) may exhibit complete unfolding when preadsorbed onto certain surfaces, such as polystyrene or hematite.
Hard proteins
are defined as proteins with high thermodynamic stability. In contrast to "soft" proteins, a hard protein is more stable to unfolding in solution in response to denaturing conditions, such as elevated temperature.
Key characteristics include:
• Adsorption Resistance: Hard proteins tend to be less adsorptive than soft proteins.
• Conformational Integrity: They are resistant to adsorption-induced unfolding or denaturation
Why do scientist study protein adsorption?
it is the fundamental event that determines the host's subsequent response to any synthetic material placed in a biological environmen
How can we control protein adsorption?
preventing nonspecific adsorption (creating nonfouling surfaces) or directing specific interactions (creating bioactive surfaces).