CHEM 18 - Module #1, #2, #3 Formulas
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Triangle formula for wavelength
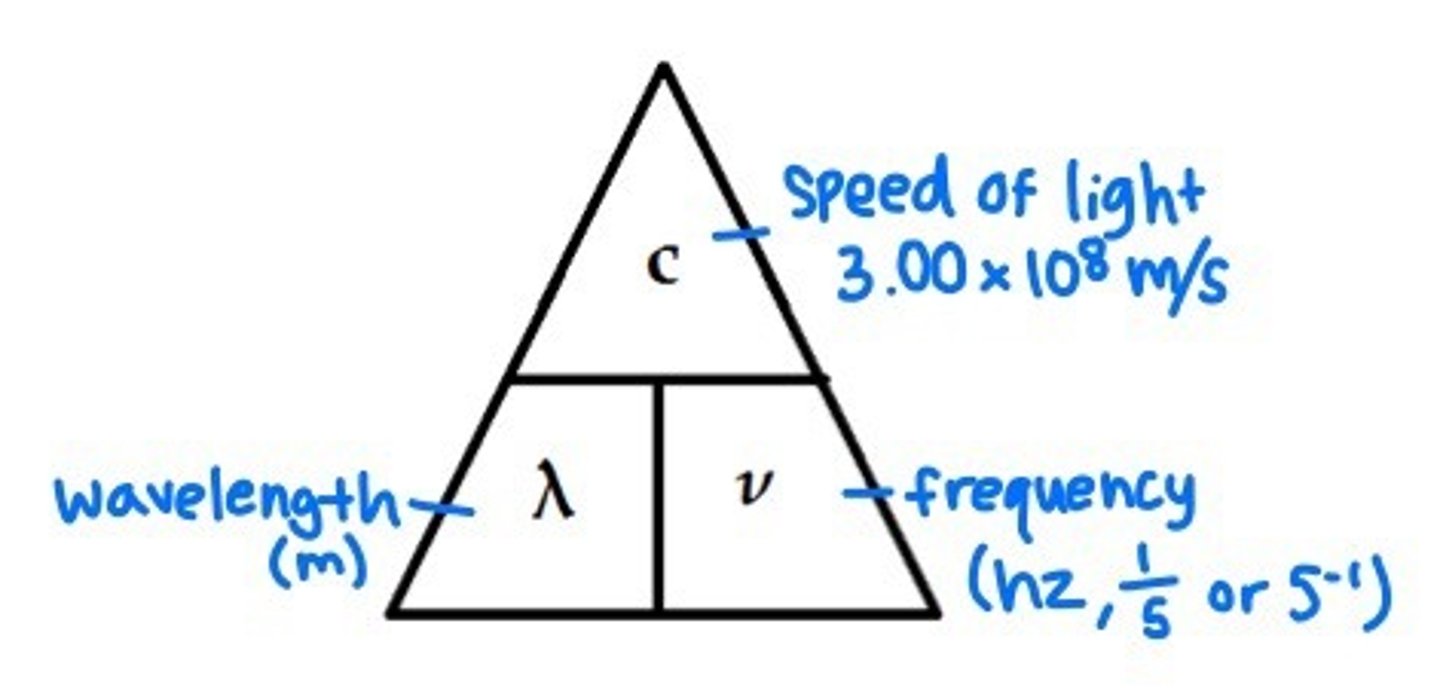
Speed of light in a vacuum
3.00 x 10⁸ m/s
Formula for energy
f in this case is v
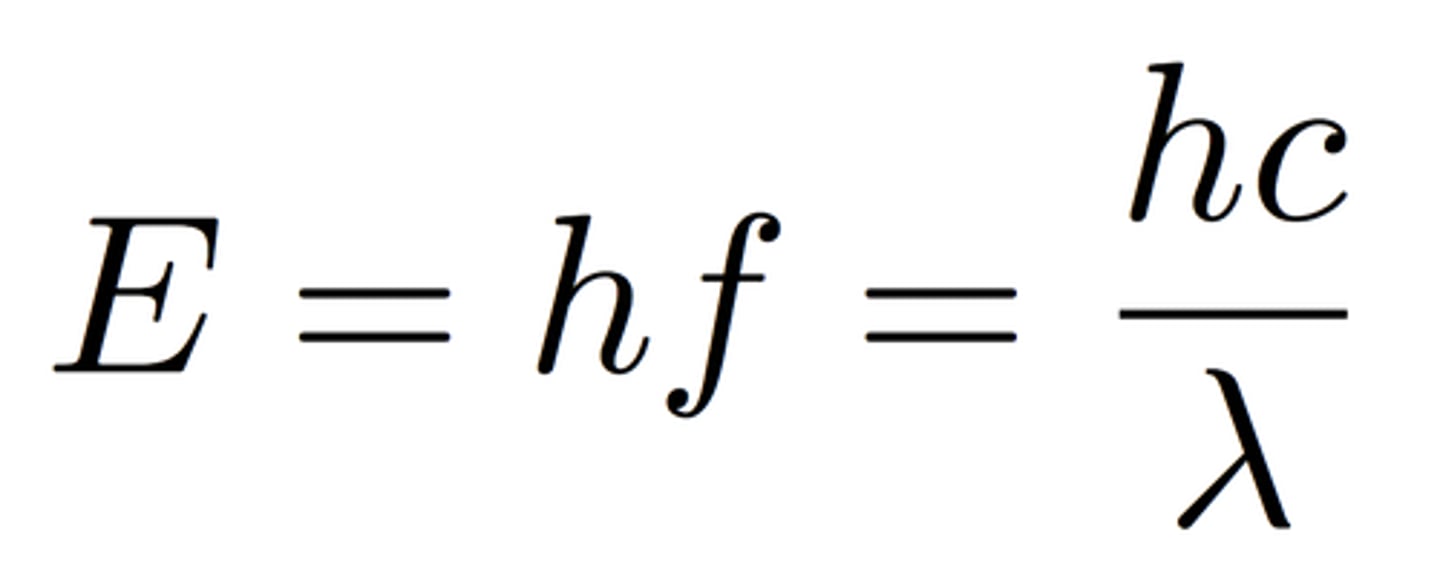
h = Planck's constant
6.626 x 10⁻³⁴ J-s
Formula for work function
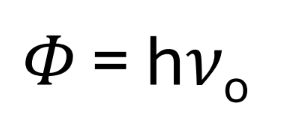
Conversion of eV to J
1 eV = 1.6 x 10⁻¹⁹J
Formula for the energy of an electron
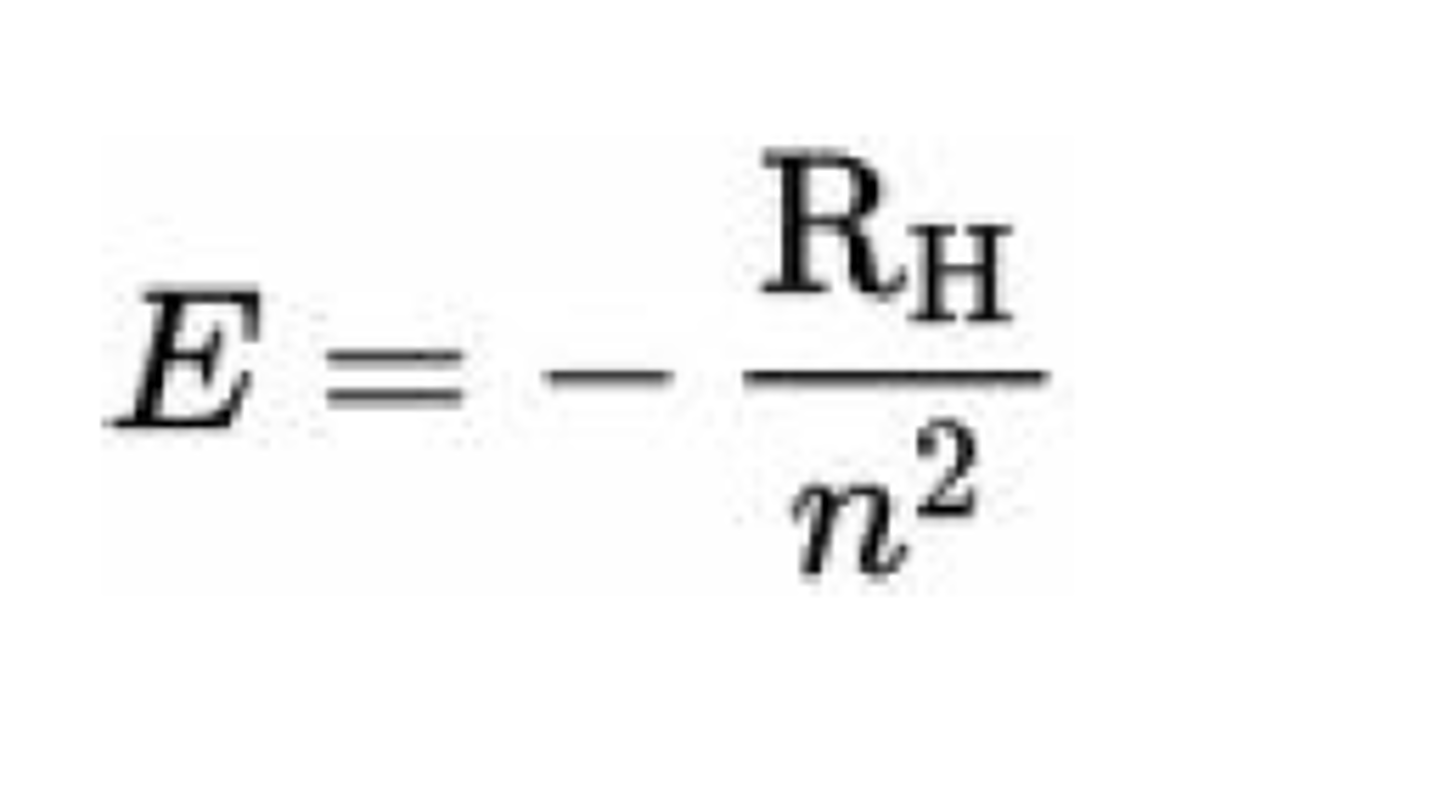
What is Rydberg's constant
RH = Rydberg's constant = 2.18 x 10⁻¹⁸ J
Formula of the wavelength of light emitted
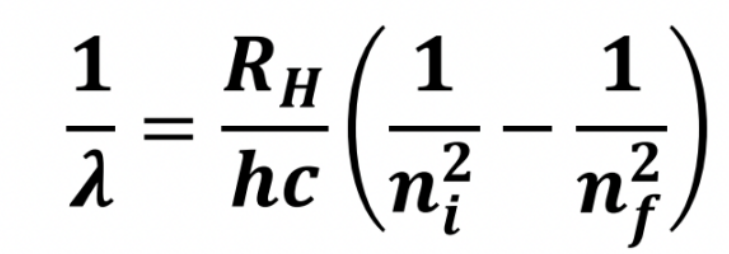
Formula for wavelength according to Louis de Broglie and what does each variable mean?
m = mass in kg
v = velocity in m/s
h = planck's constant = 6.626 x 10⁻³⁴ J-s
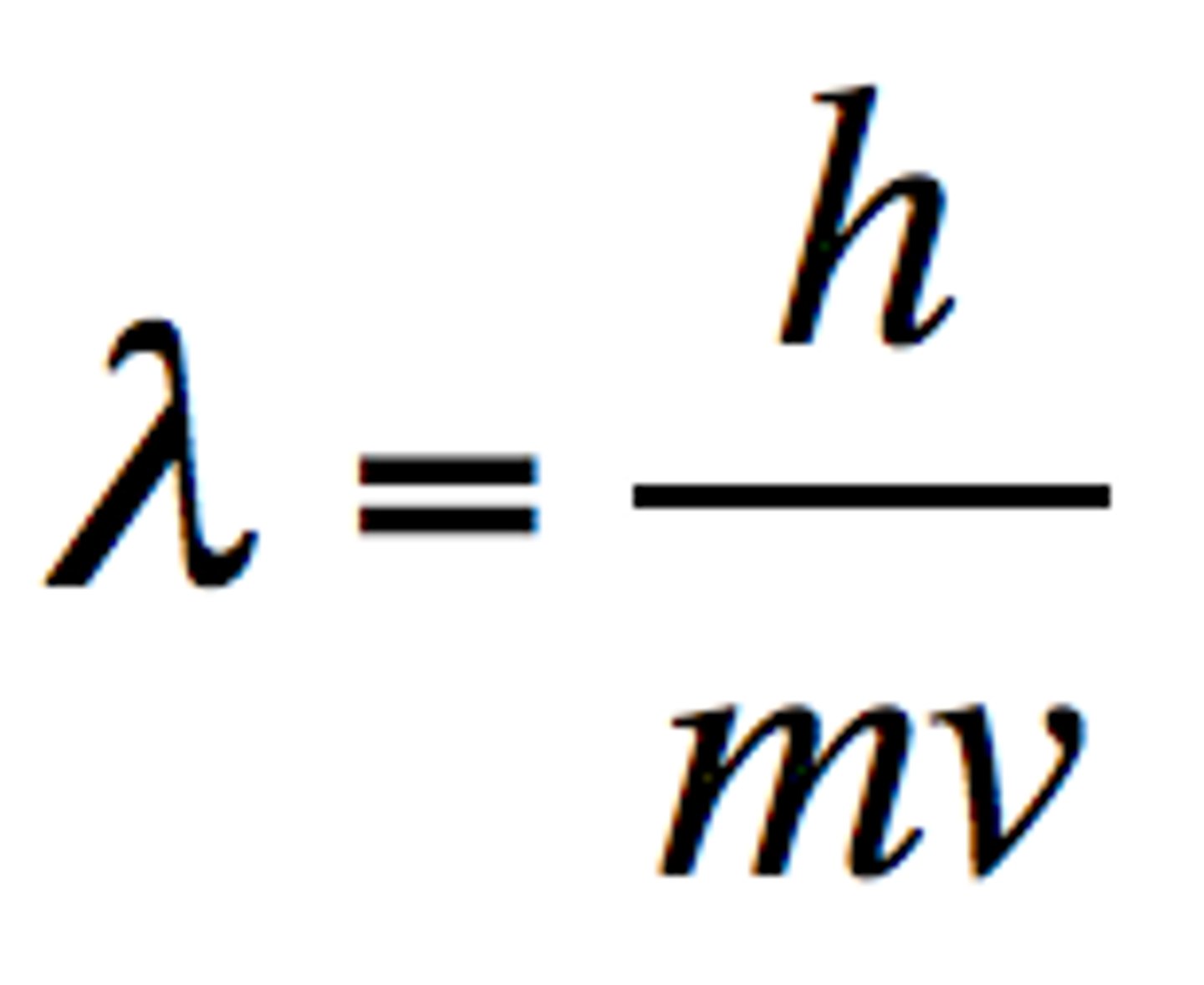
What are the conversion factors of pressure?
1 atm = 760 mmHg = 760 torr = 101325 Pa = 1.01325 bar
What are the conversion factors of volume?
1L = 1 dm³ = 1000 mL = 1000 cm³
Boyle's Law, formula and proportionality
At constant temperature and mole of the gas,
the pressure is inversely proportional to the
volume.
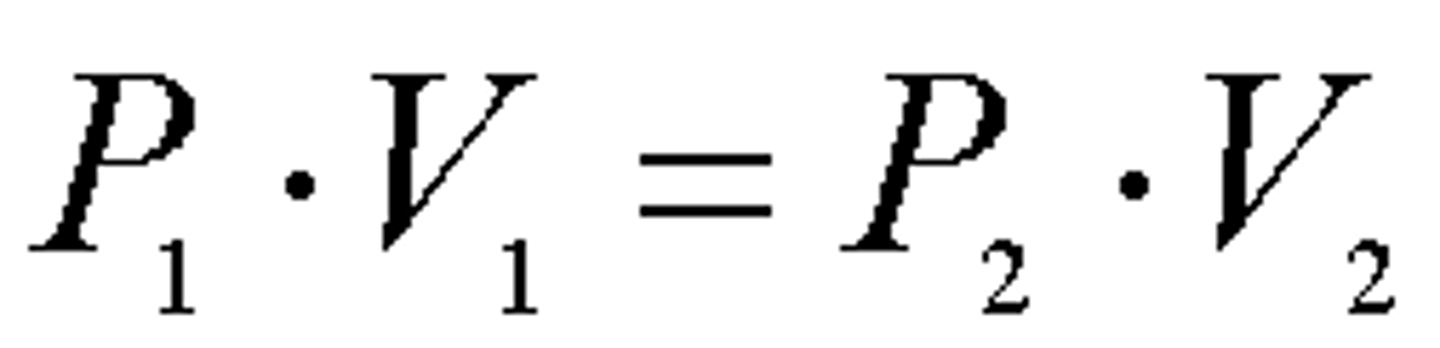
Charle's Law, formula and proportionality
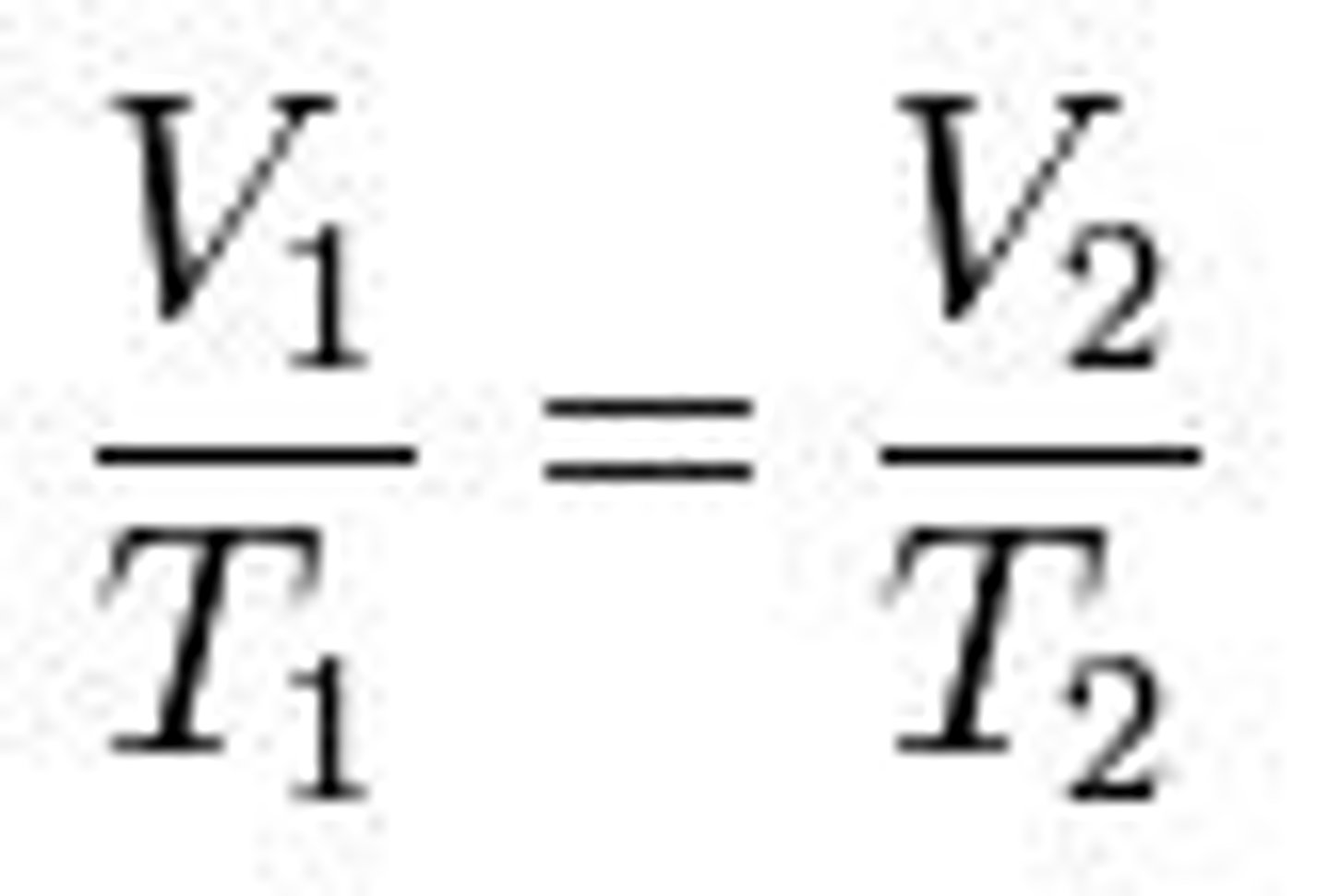
At constant pressure and number of moles, the
volume of a gas is directly proportional to its
temperature.
Avogadro's Law, formula and proportionality
At constant P and T, the V of a gas is directly
proportional to its amount in moles.
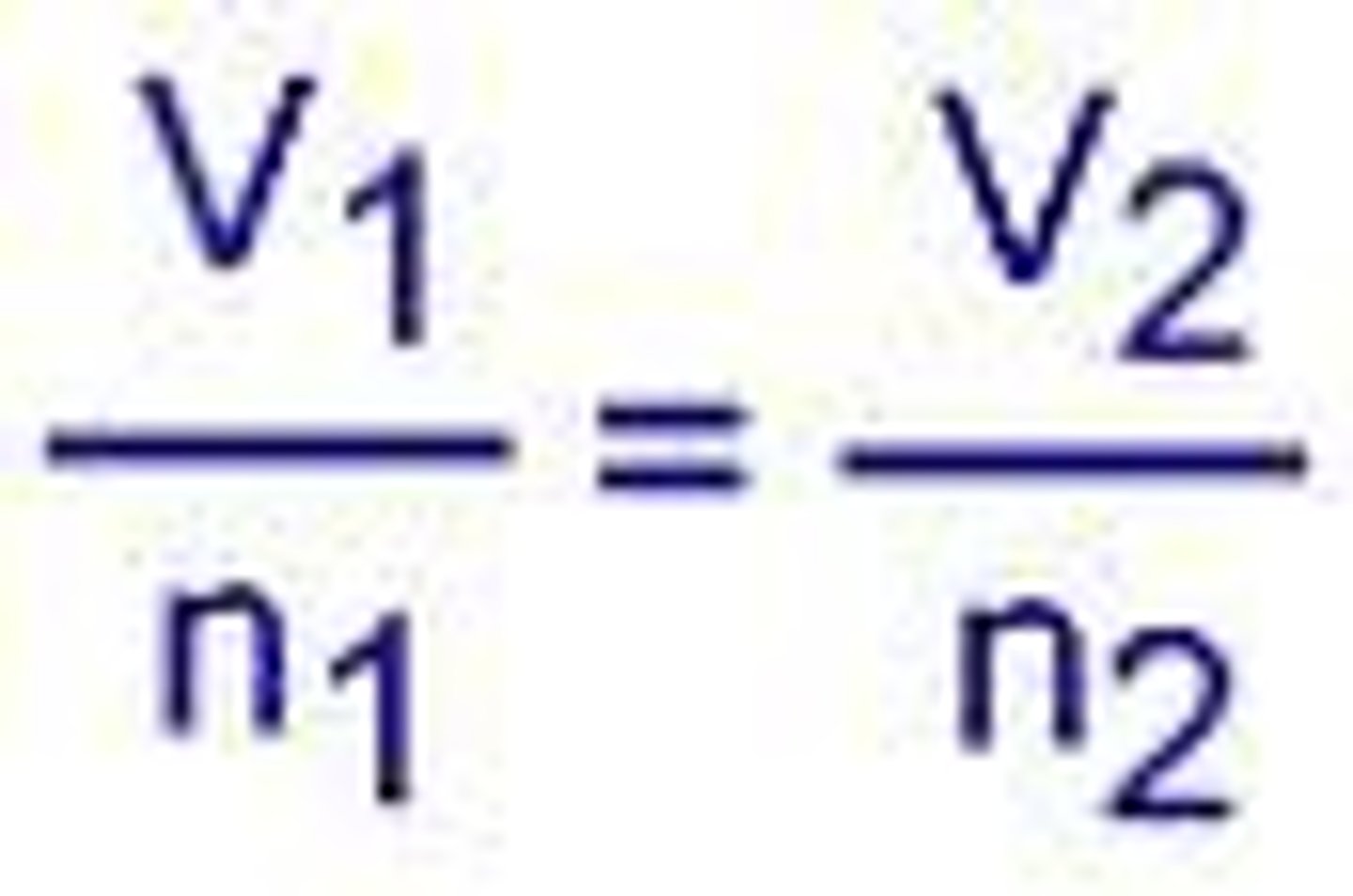
Gay-Lussac's Law, formula and proportionality
At constant V and n, the pressure exerted by a
gas is directly proportional to the Kelvin T.
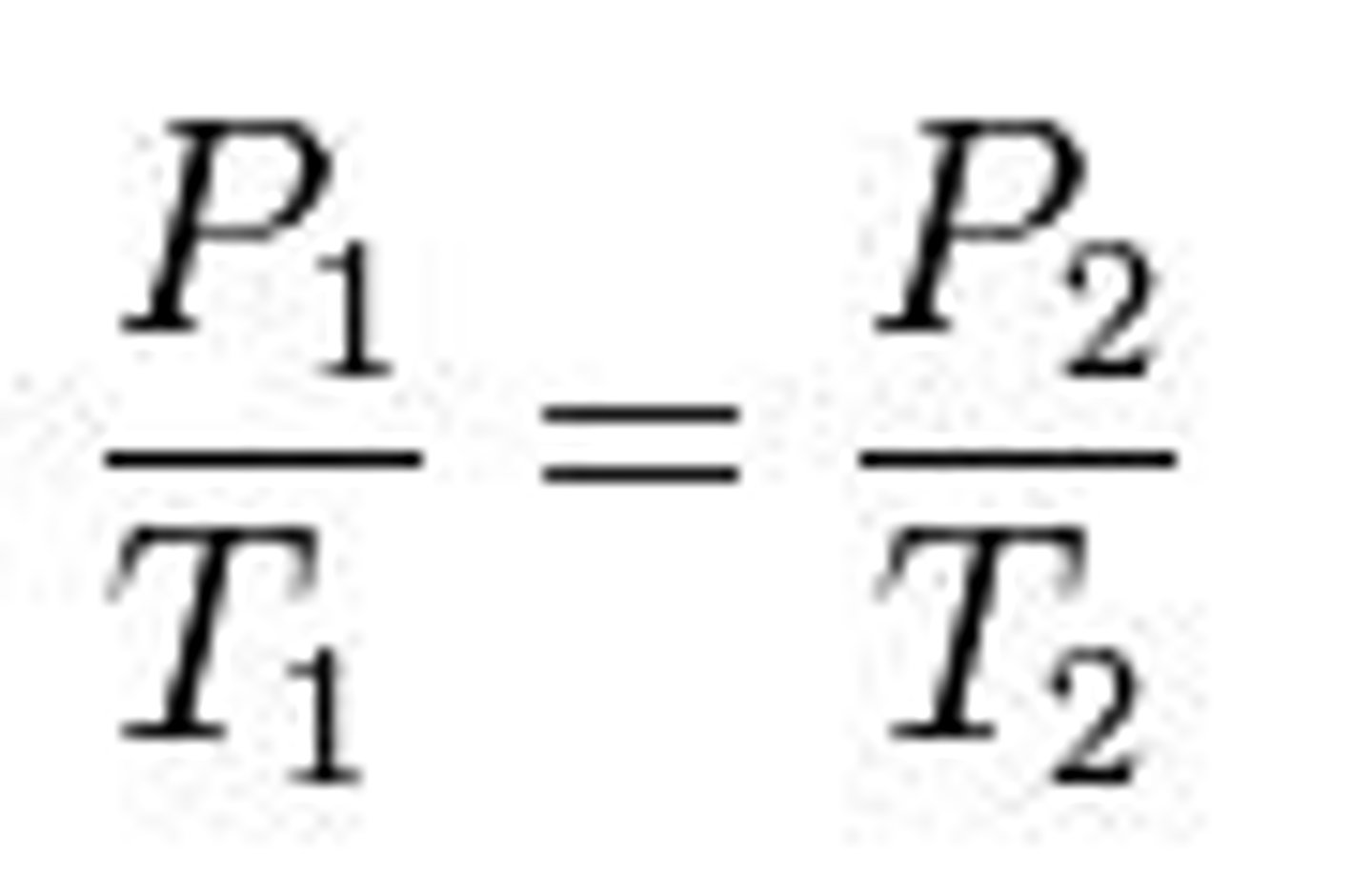
Standard Temperature and Pressure (STP)
At these conditions (__________ C° and _________ atm), 1 mole of
an ideal gas occupies a volume of _________________ Liters.
0 C° and 1 atm; 22.4
IDEAL GAS EQUATION
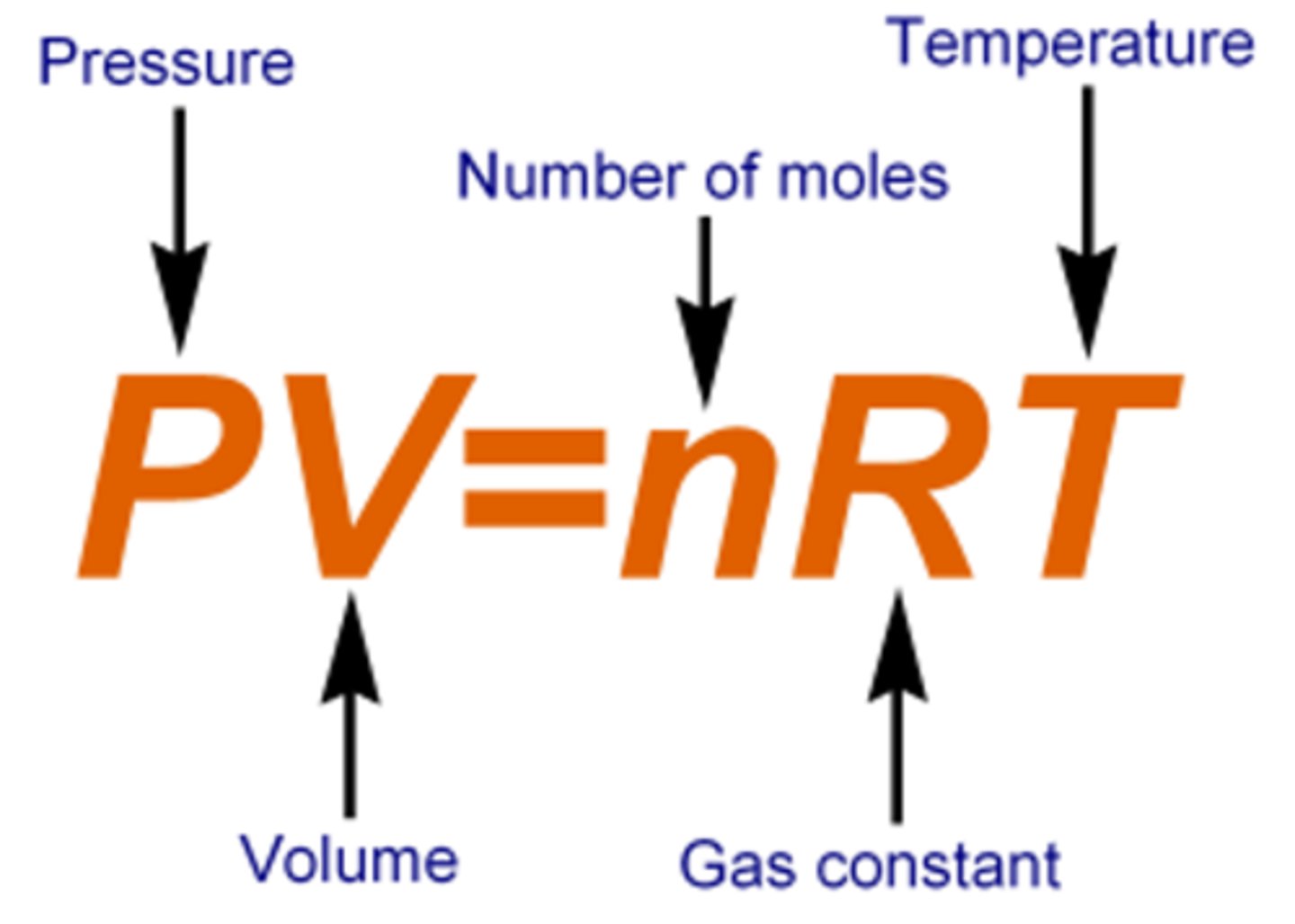
PV = nRT
Density formula
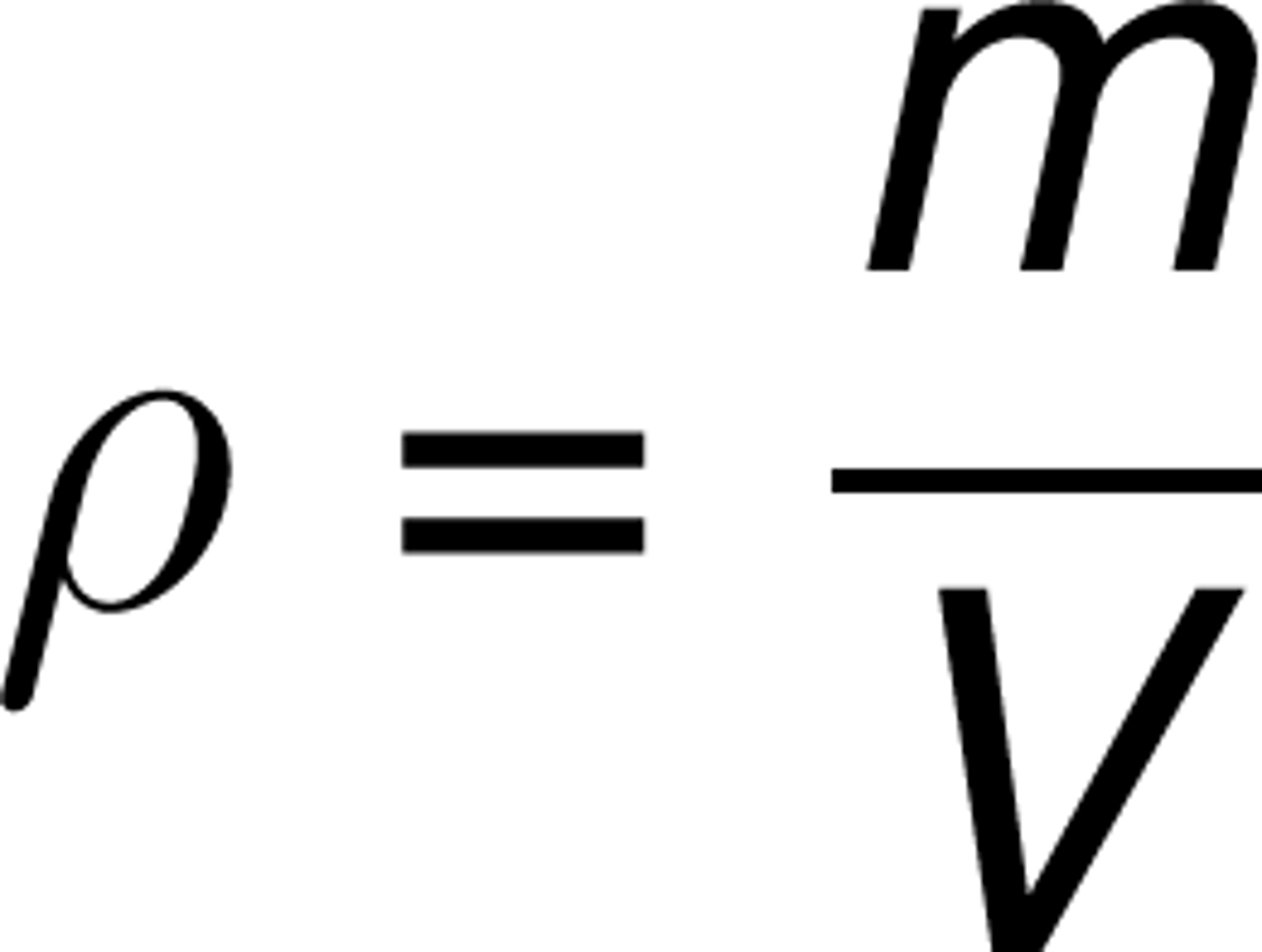
Density variation from ideal gas equation
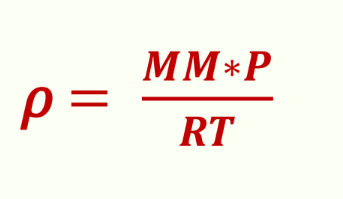
Molar mass formula
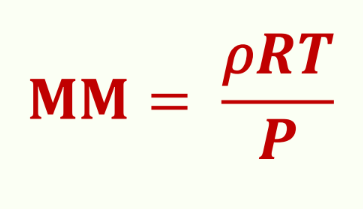
What is the gas constant?
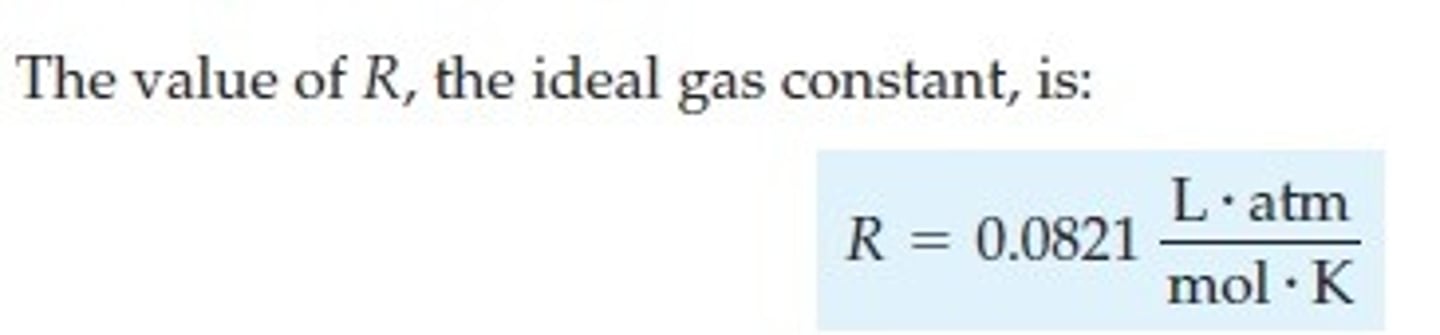
Dalton's Law of partial pressure formulas
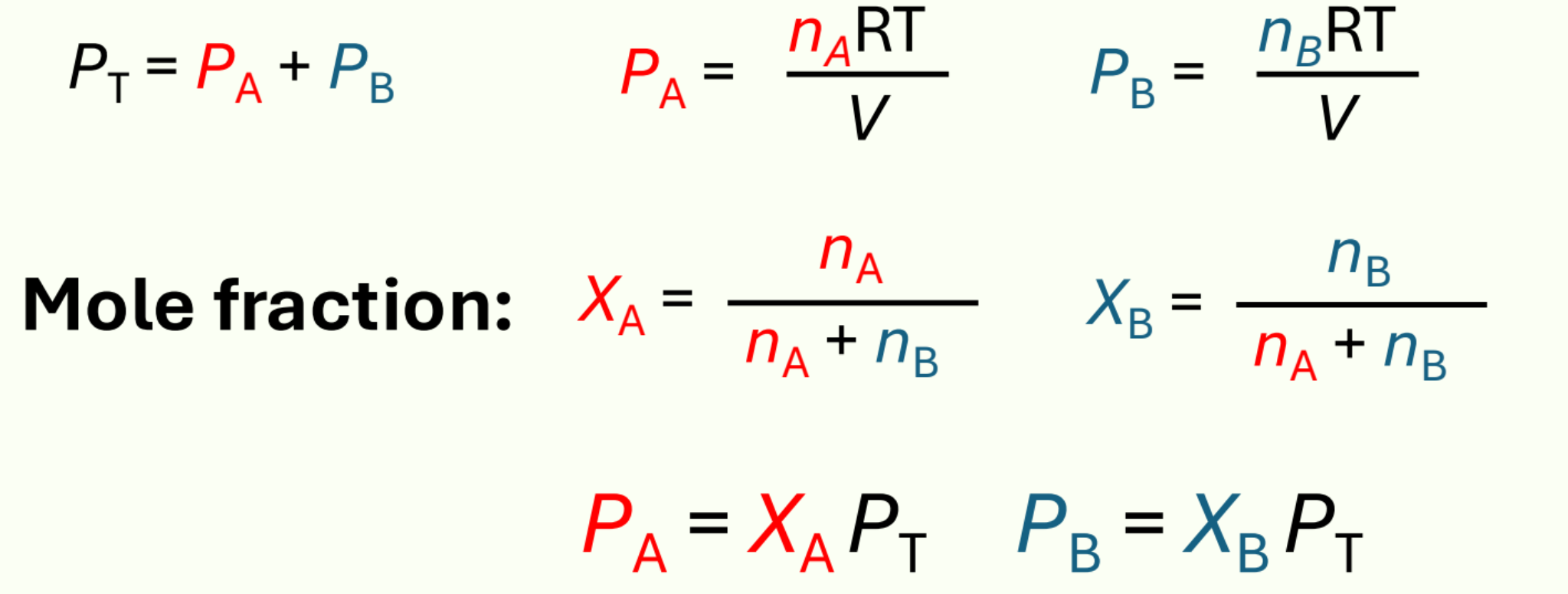
Average speed (u) formula and its values and units based on gas properties
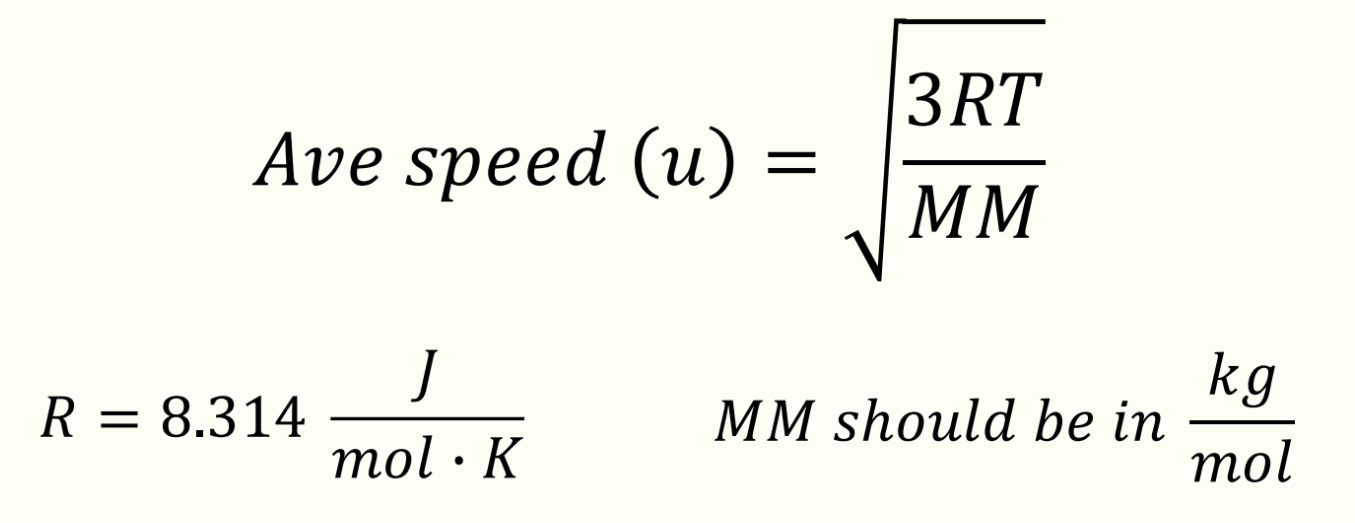
R = 8.314 J/(mol ∙ K)
MM should be in kg/mol
Unit for average speed (u)
m/s
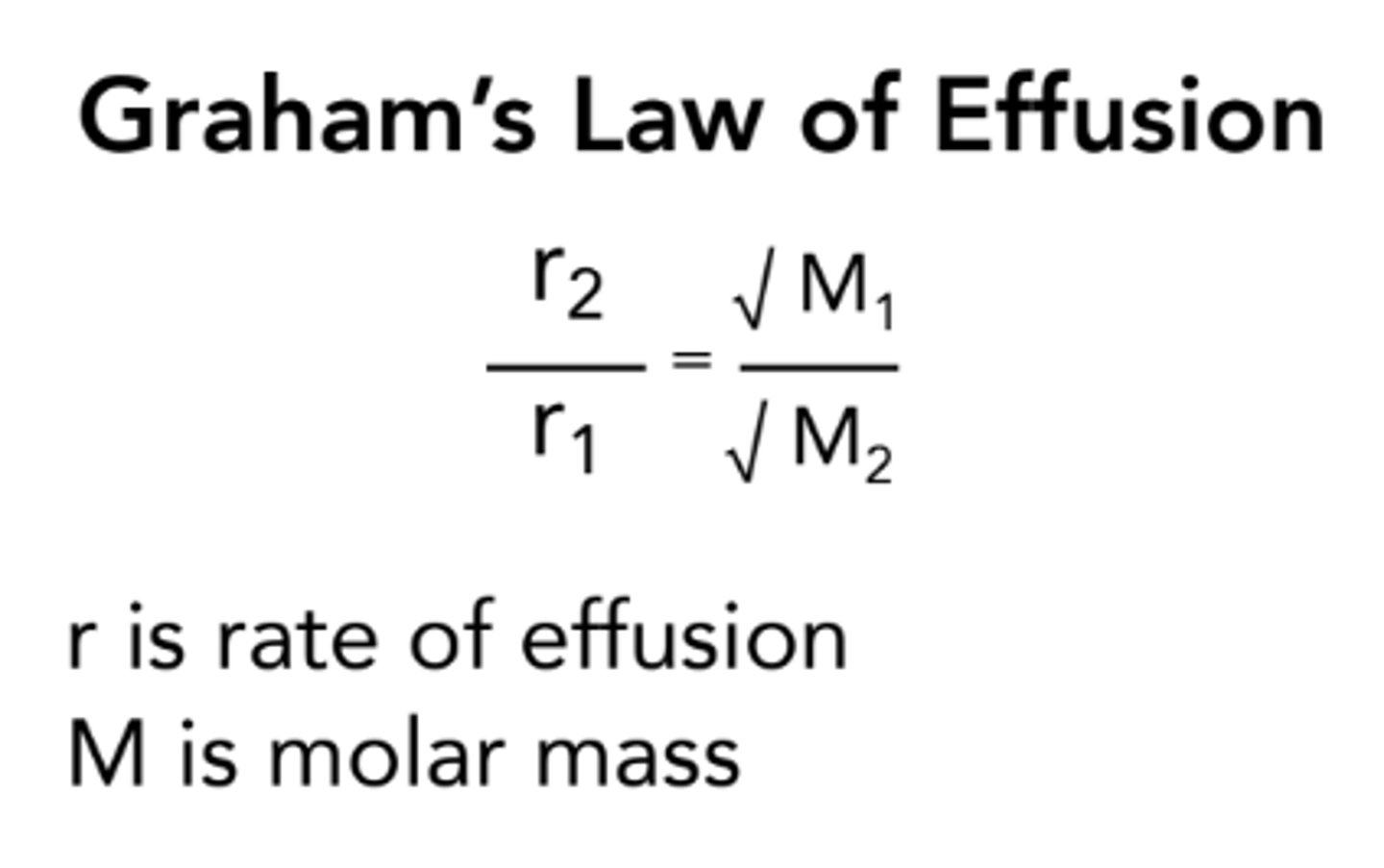
Graham's Law Formula
Compressibility factor formula
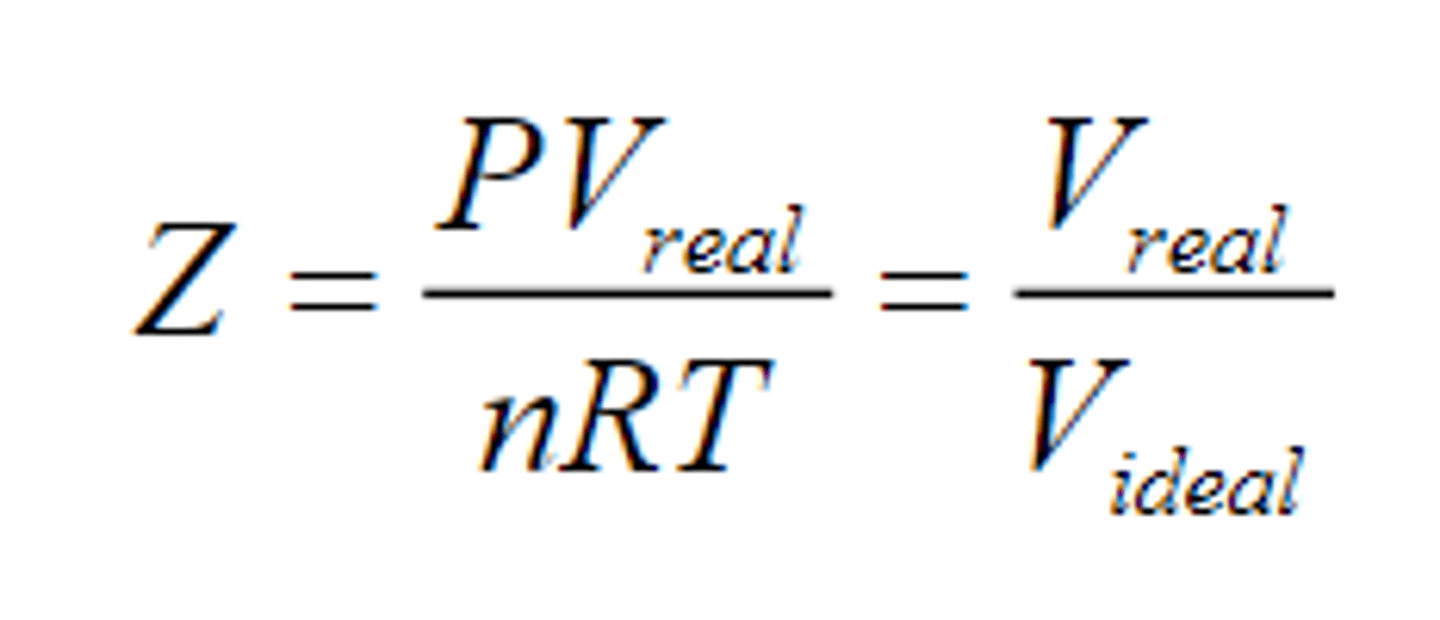
What are the conditions of the gasses based on the values calculated by the compressibility factor formula and why?
If Z = 1, it means that it is the ideal gas
If Z > 1, it means that Vreal > Videal
- because repulsion is dominating
If Z < 1, it means that Vreal < Videal
- because attraction is dominating