Monomers and polymers and Carbohydrates
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
What is a monomer ? Give examples.
Smaller units which join together to form larger molecules.
-monosaccharides (glucose, fructose, galactose)
-amino acids
-nucleotides
What is a polymer ? Give examples.
Molecules formed when many monomers join together.
-polysaccharides
-proteins
-DNA/RNA
What is a condensation reaction ?
A chemical bond forms between 2 molecules and a molecule of water is formed.
What is a hydrolysis reaction ?
A water molecule is used to break a chemical bond between 2 molecule.
Name the 3 hexose monosaccharides.
-Glucose
-Galactose
-Fructose
C6H12O6
Name the bond formed when 2 monosaccharides react.
Glycosidic bond, can be 1,4 or 1,6. Number of bonds to number of reacting molecules is 1:2.
Name the 3 disaccharides. Describe how they form.
-glucose + glucose → maltose
-glucose + galactose → lactose
-glucose + fructose → sucrose
All + (water)
C12H22O11
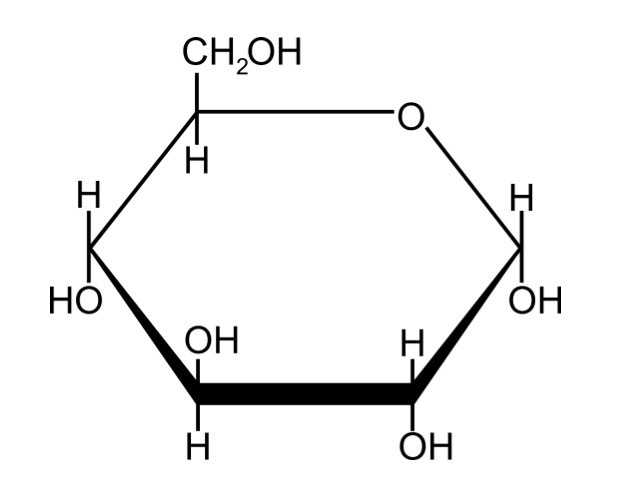
Alpha Glucose
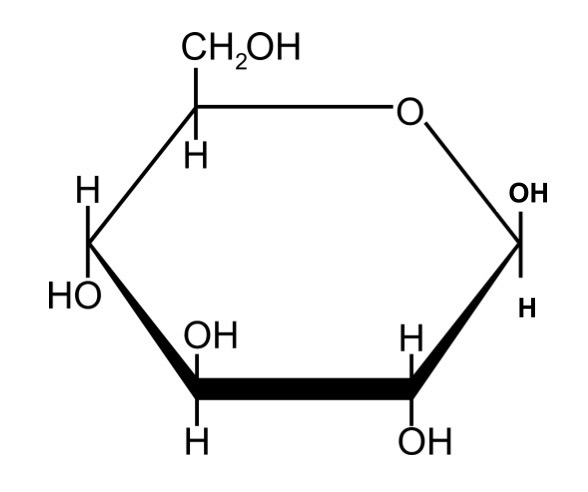
Beta Glucose
Describe the function of starch and how it relates to its function.
Function - storage molecule of alpha glucose in plant cells.
Structure:
-large so no osmotic effect on cells
-insoluble so doesn’t diffuse out of cells
-made from amylose with 1,4 glycosidic bond, and helix with intermolecular H bonds so compact
-made from amylopectin with 1,4 and 1,6 glycosidic bonds meaning its branched so many terminal ends for hydrolysis into glucose
Describe the function of glycogen and how it relates to it function.
Function- main storage polymer of alpha glucose in animal cells (also in plant)
Structure:
-has 1,4 and 1,6 glycosidic bonds so its branched meaning many terminal ends are exposed for hydrolysis
-insoluble so no osmotic effect and doesn’t diffuse out of cells
-compact
Describe the function of cellulose and how it relates to its function.
Function - polymer of beta glucose and gives rigidity to plant cell walls (prevents bursting under turgor pressure, hold stem up)
Structure:
-has 1,4 glycosidic bonds so its a straight unbranched molecule
-alternate glucose molecules are rotated 180 degrees
-there are H bonds crosslinks between parallel strands from microfibrils leading to high tensile strength
Describe the Benedict test for reducing sugar.
Add an equal volume of Benedict reagent to the sample
Heat the mixture in a water bath to 100 degrees for 5 mins
Positive result (reducing sugar present) : blue solution → brick-red precipitate forms
Describe the Benedict’s test for non-reducing sugar.
Negative result when Benedict’s test carried out (remains blue)
Hydrolyse the non-reducing sugar (sucrose → fructose + glucose) by adding hydrochloric acid and heating in a boiling water bath for 5 mins.
Neutralise the mixture using sodium carbonate solution and reattempt Benedict’s test as usual.
Describe the test for Starch
Add iodine solution
Positive result: orange → blue-black
How could colorimetry give qualitative data for the presence of sugars and starch.
Make standard solutions with known concentrations (dilution series)
Record absorbance or transmission
Plot calibration curve with absorbance/transmission on the x-axis and concentration on the y-axis
Record the absorbance/transmission of unknown sample, and use the calibration curve to read off concentration.