2.6 - Halogenoalkanes
1/16
Earn XP
Description and Tags
In this unit we will learn about the structure of halogenoalkanes, including hydrolosis of halogenaolkanes and their uses.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Halogenoalkane
An alkane with at least one halogen in place of a hydrogen atom.
Halogenoalkane general formula.
CnH2n+1X
Nucleophile
A species with a lone pair of electrons that can be donated to an electron deficient centre to form a new covalent bond, E.g. halogen anions, OH- ions.
Test for halogen in haloalkane.
Add NaOH(aq)
Add HNO3 and AgNO3(aq)
Observe colour change of precipitate
Chloride precipitate colour
White
Bromide precipitate colour
Cream
Iodide precipitate colour
Yellow
Nucleophillic substitution
Preparation of alcohols by substitution reactions of halogenoalkanes.
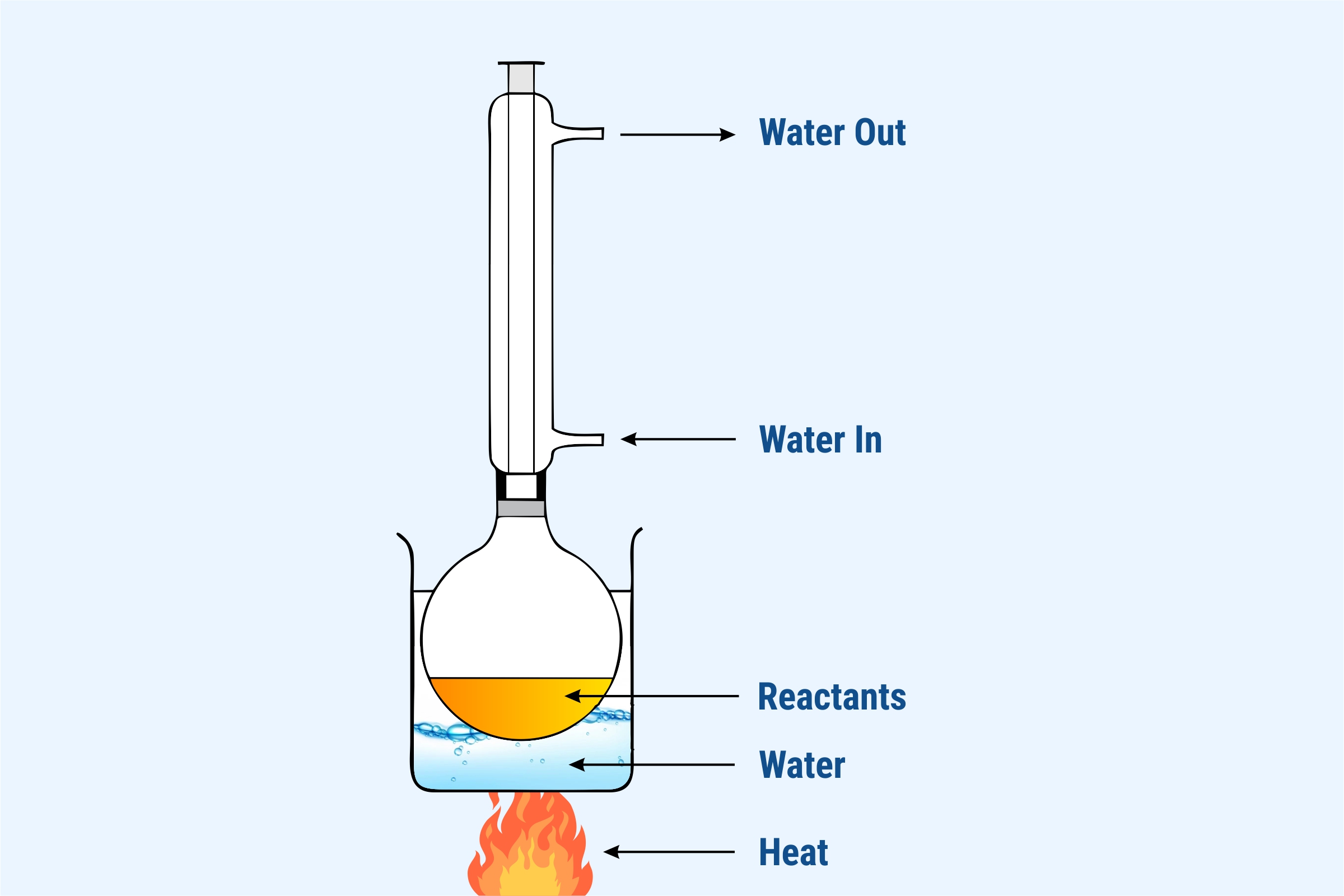
Conditions for nucleophillic substitution
Heat under reflux.
Electronegativity
The ability of an atoms, when in a covalent bond to pull the lone pair of electrons towards itself.
Bond strength
To substitute the halogen you need to break the C-X bond. the strongest is C-Cl so it is the hardest to break.
Test for the rate of hydrolysis (Nucleophilic substitution)
Measure time taken for silver halide precipitate to form.
Elimination reactions (No water)
A small molecule is lost to form a double bond. (alkene)
Conditions for elimination reaction.
Concentrated sodium hydroxide.
Ethanol (E for elimination, E for ethanol)
Heat.
Uses of halogenoalkanes.
Solvents
Anaesthetics
Refrigerants
Halogenoalkanes disadvantages
CFC’s - cause holes in ozone layer
In ozone layer CFC’s encounter UV light which breaks the C-Cl bond, making a chlorine free radical.