Nomenclature, ligands, and classification
1/15
Earn XP
Description and Tags
https://chem.libretexts.org/Courses/Ripon_College/CHM_321%3A_Inorganic_Chemistry/06%3A_Organometallic_Chemistry/6.02%3A_Nomenclature_Ligands_and_Classification
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
how to incorporate hapiticity into naming compounds
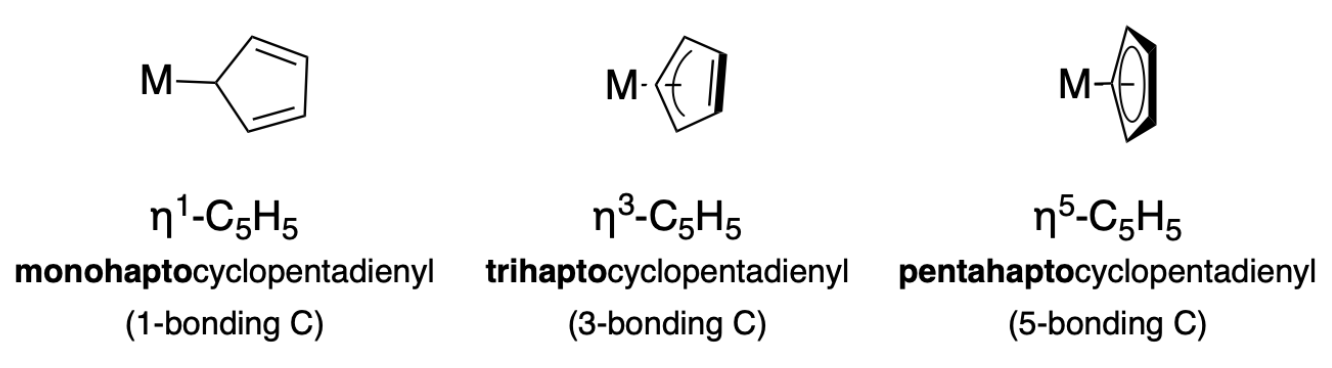
Hapicity refers to the number of attachment points a ligand has to a central atom in a coordination complex. When naming compounds, it is indicated by the prefix 'mono-', 'bi-', 'tri-', etc., followed by “hapto” before the ligand's name, followed by the coordination number.
name + ligand classification
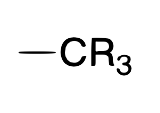
allyl
X
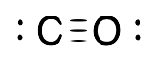
name + ligand classification
carbonyl
L
name + ligand classification
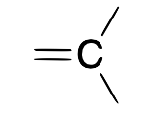
carbene (alkylidene)
X2
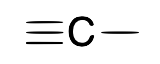
name + ligand classification
carbyne (alkylidyne)
X3
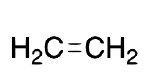
name + ligand classification
ethylene
L
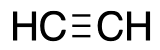
name + ligand classification
acetylene
L
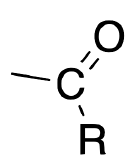
name + ligand classification
acyl
X

name + ligand classification
π-allyl (C3H5–)
X for η1
LX for η2

name + ligand classification
cyclopropenyl (cyclo-C3H3)
X for η1
LX for η3

name + ligand classification
cyclobutadiene (cycle-C4H4)
L2 for η4
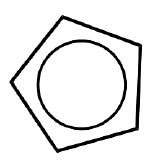
name + ligand classification
cyclopentadienyl (cyclo-C5H5, Cp)
X for η1
LX for η3
L2X for η5
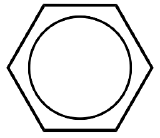
name + ligand classification
benzene
L3 for η6
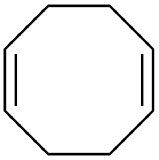
name + ligand classification
1,5-cyclooctadiene (1,5-COD)
L2 for η4
metal hitting at center of double bond: L or X
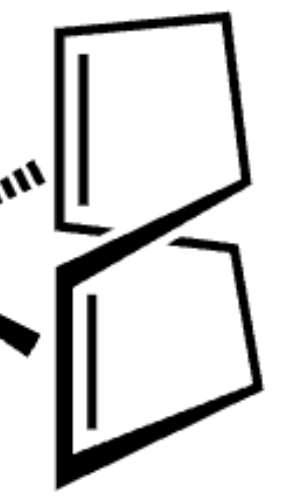
L type
M=[ligand] L or X?
X2