Actin Filaments
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
78 Terms
9 intracellular locations of actin filaments
Microvilli
Cell cortex
Adherens belt
Filopodia
Lamellipodium
Stress fibres
Phagocytosis
Moving endocytic vesicles
Contractile ring
How do actin filaments facilitate cell movement?
Actin filaments extend the lamellipodium and pull the cell forward
The leading edge pushes forward and stress fibres pull up the rear
Extension
Polymerize actin and protrude
Focal adhesion
Adhesion
Extend lamellipodium
Translocation
Pull cell body forward
De-adhesion and endocytic recycling
Phases of actin polymerization
0. Actin is diffusing around in solution: G-actin (globular actin is a protein by itself)
Nucleation: 3 actin subunits collide in such a way that they collide and form a nucleus for the polymerization of actin (Slow)
Elongation: Protein polymerizes
Actin subunits collide with the nucleus
F-actin: filamentous actin
Steady state: New actin keeps colliding
(+) end of actin filament's on rate constant
12 µM-1s-1
(+) end of actin filament's off rate constant
1.4 s-1
(+) end of actin filament's critical constant
C+c = 0.12 µM
(-) end of actin filament's on rate constant
1.3 µM-1s-1
(-) end of actin filament's off rate constant
0.8 s-1
(-) end of actin filament's critical constant
C-c = 0.60 µM
What happens to the concentration of free actin as a polymer grows?
The concentration of free actin decreases
What happens when the concentration of free actin decreases to C-c?
The (-) end reaches equilibrium
Why does the (+) end of the polymer continue to grow even after the (-) end reaches equilibrium?
Because the concentration of free actin is still above C+c for the (+) end
What happens when the concentration of free actin drops below the C-c of the (-) end?
The (-) end starts shrinking to maintain equilibrium
Actin is an ______ that drives cell motility
Actin is an ATPase that drives cell motility
How do actin filaments contribute to the lamellipodium?
Directly behind filopodia
How do actin filaments contribute to stress fibres?
At the back end of the cell
What features of actin filaments can cells control?
LANBO
Length
Angle
Number
Bundling
Orientation
Structure of microfilaments
Built by actin
Two stranded polymer
Forms thick bundles: stress fibres
7-9 nm thick
Actin's 2-stranded helical filament structure
36 nm for one helical pitch
Polarized filaments
(+): smooth end
(-): notched end
How do actin filaments contribute to the adherens belt?
Seals from fluids leaking
How do actin filaments contribute to the contractile ring?
When cells are undergoing cytokinesis, they allow for the cell to cleave into two cells
How do actin filaments contribute to the cell cortex?
They line the outer edge of the cell
How do actin filaments contribute to the filopodia?
At the leading edge, driving motion
How do actin filaments contribute to phagocytosis?
When the cell is engulfing, they outline the site of entry
Why is the off rate constant lower than the on rate constant on the (+) end?
Probability of fall off is driven not by concentration but by random kinetic events like ion and water colliding with the filament, which is less likely
What is the critical constant?
C+c is the concentration at which on and off events are equal
What is the process called when the (+) end grows while the (-) end shrinks in a polymer?
Treadmilling
In actin treadmilling, the _____ stays the same, the _____ grows and the ____ shrinks.
In actin treadmilling, the length stays the same, the (+) end grows and the (-) end shrinks.
Formula to calculate net growth
Net growth = subunits (+) - subunits (-)
Net growth = kON [subunits] - kOFF
(+): on rate const. * [subunits]
(-): off rate const.
Formula to calculate Cc
Cc = kOFF / kON
Net growth = 0
5 Main actin regulators
Formins
Cofilin
Capping protein
Arp 2/3
⍺ - actinin
Formins
Grow the filaments by accelerating the rate of actin growth
Cofilin
Cut the filament / fragment it
Capping proteins
Stop it from growing and polymerizing
Arp 2/3
Make branched network
⍺ - actinin
Cross-link the filaments
Function of cross-linkers at binding domains
Regulate the spacing and orientation of resulting bundle
Cross-linkers at binding domains
Fimbrin
⍺ - actinin
Spectrin
Filamin
Fimbrin
Local adhesion
Location: microvilli, filopodia, focal adhesions
⍺ - actinin
Dimer
Each polypeptide has 1 actin binding domain
Location: stress fibers, filopodia, muscle Z line
Spectrin
Spectral actin network
Heterotrimer, dimer
Location: Cell cortex
Filamin
Singular polypeptide
Flexible kink
Location: Leading edge, stress fibres, filopodia
How does actin allow for pathogenic bacteria to move?
Actin makes a comet tail for the bacteria to move around to break out of the cell and infect other cells
Listeria “comet tails” are nucleated by the Arp 2/3 complex
How does Arp 2/3 function?
Mimics an actin nucleus (when polymerizing)
Dimer comes together when activated on the daughter filament
Attaches at 70º
Brownian ratchet: Presses up against the PM until it gets stuck
What activates the Arp 2/3 complex?
WASp delivers the actin monomer to Arp 2/3
What are the domains of WASp?
WH2 domain
A domain
C domain
What does the WH2 domain in WASp do?
Binds to the actin monomer
What does the A domain in WASp do?
Acidic domain binding the Arp 2/3 complex
Conformational change bringing it together
What main challenges does Lamellipodia run into during activity?
Running out of actin
Preventing futile polymerization
How does the cell make sure that it doesn’t run out of actin?
It keeps the concentration of actin relative to the critical constant
How does the cell make sure that there is no futile polymerization?
Don’t grow actin far away
Don’t want actin polymerizing at the (-) end
What protein aids the complications of Lamellipodia? How does it function?
Prolifin
Recycles actin
Maintain high concentration
Blocks (-) end polymerization
ADP-G actin refreshed and accelerates rate of ATP exchange
What protein severs actin filaments?
ADF/Cofilin
At the (-) end
Unwinds the actin and creates instability
Together, what proteins accelerate actin filament treadmilling?
Cofilin: depolarizes
Profilin: recharges
Thymosin - β4: sequesters actin in reverse
What proteins prevent futile polymerization? How?
Capping protein
Caps the actin filament so that new actin doesnt bind
(+) end: Cap Z
(-) end: Tropomodulin
How do formins polymerize linear actin filaments?
Dimer sits on top of actin
Moves up as actins are added to the filament
FH2: Domains sit at the end
FH1: Long slender arms that bind to profilin actin — feeding
Adds >10,000 monomers before detaching
What is myosin?
Myosin is a motor protein that produces force by converting chemical energy into mechanical energy
What experiment demonstrates myosin’s ability to translocate actin filaments?
In experiments where myosin heads attach to a glass slide, actin filaments move, with the (-) end moving forward as myosin heads try to move towards the (+) end
What are the three classes of myosin? Step sizes?
Class I: 10-14 nm
Class II: 5-10 nm
Class V: 36 nm
What is the primary function of Class I myosin?
Class I myosin is involved in membrane association with the actin cytoskeleton and endocytosis
What is the primary function of Class II myosin?
Class II myosin is responsible for contraction
What is the primary function of Class V myosin?
Class V myosin binds to organelles like vesicles and facilitates their transport
What does non-muscle myosin-II do to actin filaments?
Non-muscle myosin-II forms bundles that pull actin filaments inward. Filaments at the ends create inward directional force that pushes actin inward.
How do myosin-II bundles contribute to cellular movement?
Myosin-II bundles contract actin arrays, enabling cytoskeletal rearrangement necessary for movement.
What is Dictyostelium, and why is it significance?
Dictyostelium is an amoeba that aggregates with other amoebae during starvation, demonstrating cellular communication and collective behavior.
How does Dictyostelium respond to starvation?
Sends stress signals that prompt other amoebae to produce fruiting bodies, allowing some members to relocate to areas with more food.
What chemical signal does Dictyostelium use for movement, and how does it work?
Dictyostelium uses cyclic AMP (cAMP). Cells send out cAMP signals, and other amoebae move toward the higher concentration of cAMP
Why do myosin heads move toward the (+) end of actin filaments?
Myosin heads generate mechanical force by hydrolyzing ATP, propelling them toward the (+) end for filament sliding
What happens to actin filaments when myosin-II bundles contract?
Contraction of myosin-II bundles pulls actin filaments inward, creating mechanical force for cellular functions like cytokinesis
What is the relationship between myosin-II and cellular contraction?
Myosin-II’s ability to form bundles and pull actin filaments inward enables the contraction of actin arrays, a key process in cellular movement and division.
What are the 3 types of Rho family GTPase? In short, what do each do?
Dominant active Rho: actin —> stress fibers
Dominant active Rac: Causes cells to grow lamellopodia
Dominant active Cdc42: produce filopodia
How do Rho GTPase translate outside signals into changes in the actin cytoskeleton?
A receptos binds to an extracellular signal (e.g. cAMP)
The Rho fam. GTPase is initially in an inactive GDP-bound state
The receptor interacts with a GEF
(GDP —> GTP on Rho) to go into active stateRho undergoes a conformational change: interact with effector proteins
Effector proteins (e.g. formins and Arp 2/3) regulate the actin cytoskeleton
To ensure precise control, cells need to toggle the activity of Rho proteins on and off
To deactivate Rho, GAP triggers the hydrolysis of GTP —> GDP
How does Rho activate formins?
Relieves formin auto-inhibition. RBD blocks FH2 when folded, but the binding of Rho unfolds.
How does Cdc42 activate Arp 2/3?
Opens WASp, activate Cdc42 interacts with WASp and RBP, activating Arp 2/3
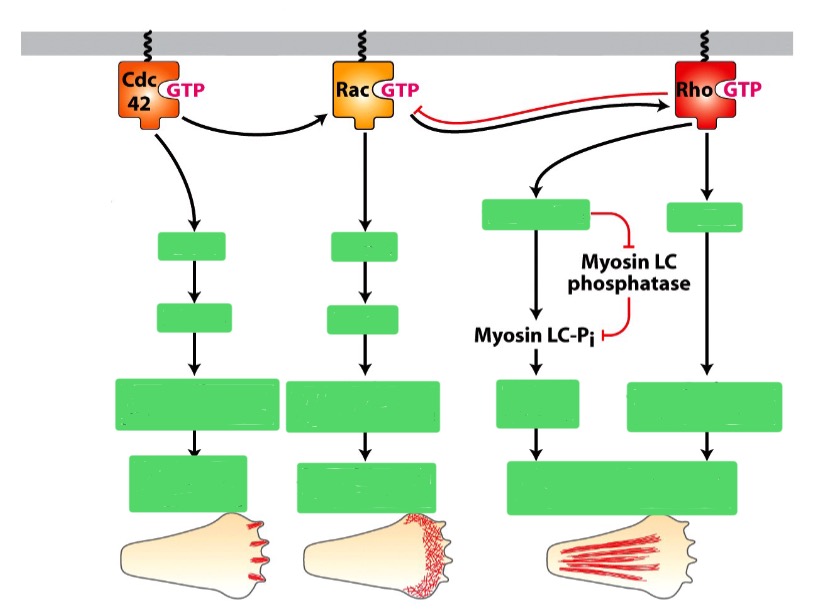
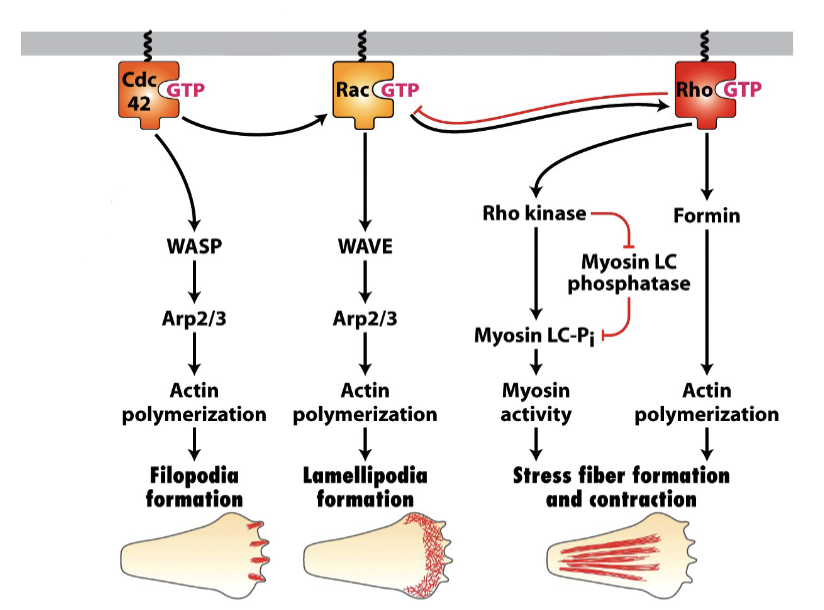
How do extracellular signals in cells create zones of different Rho activity?
Cdc42 activation at front
Front/Leading edge: Rac activation leads to Arp 2/3 activation
Actin filaments assemble to treadmilling
Back: Rho activation leads to Myosin II activation
Contraction of myosin II filaments in both stress fibres and cell cortex
Chemotactic gradient
Concentration of cAMP is higher at the leading edge
What mechanism in the cell allows for the back to become “Rho exclusive”?
Wave of Rac propagates back —> Rho —> stress fibres
Rho pushes back on Rac —> back = Rho exclusive