Practical PART - parasitology
1/24
Earn XP
Description and Tags
del 1 - first 3 cards - general info on the examination methods, then exam qs. (FINISHED)
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
How can we perform coprological examination - flotation methods?
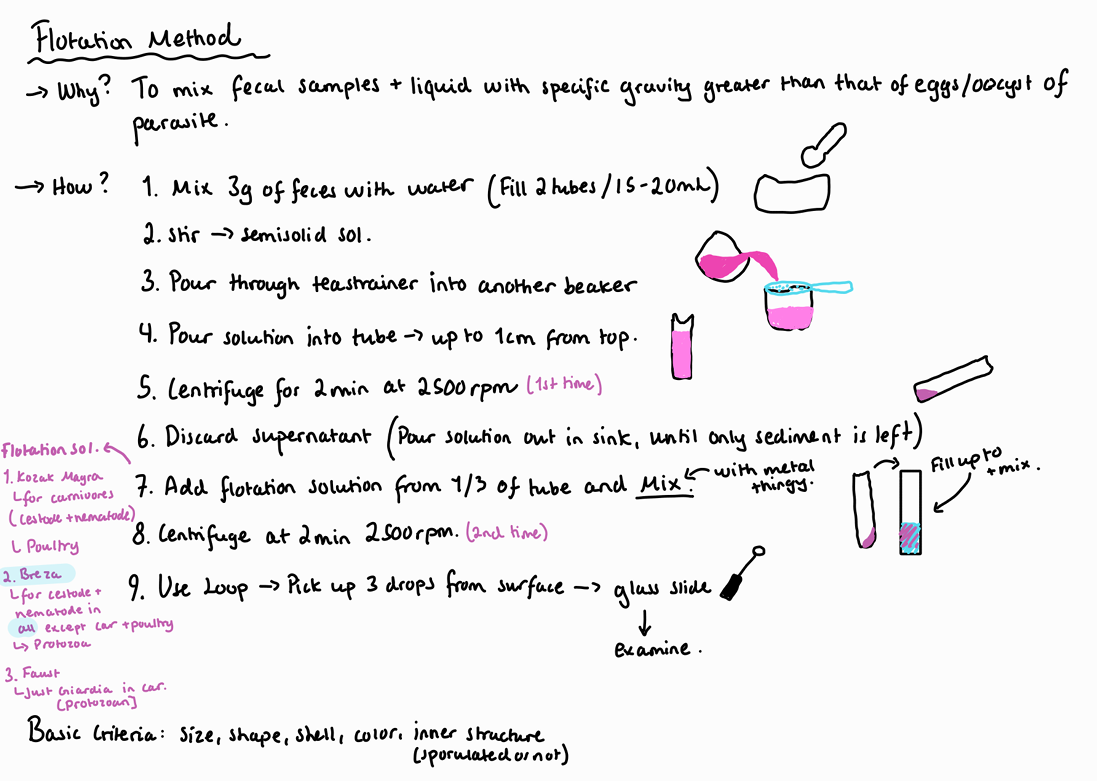
Feces can be refrigerated until ready for examination. Or helminth eggs may be preserved with formaline.
Kozàk Mágrová
Coccideas in Car
Cestodes and nematodes of Car
Poultry
Breza
Cestodes and nematodes in all animals except Car and poultry
Protozoa too
Faust
Just giardia in Car
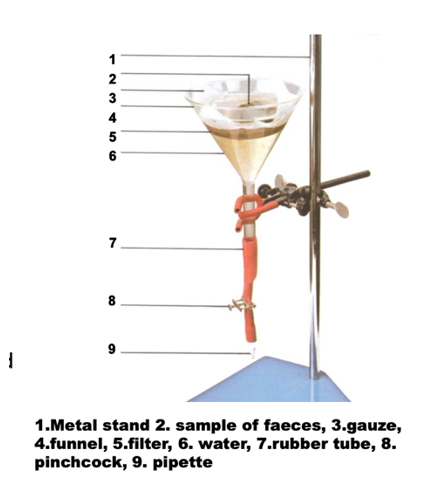
How to perform Coprological examination - Sedimentation methods?

NB: after filtration → tube/beaker → Add water (100ml) → wait 10min → remove supernatant (pour out in the sink) and add water up to previous level → remove after 5 min → repeat until supernatant is clear → put sediment on watch glass.
How to perform Coprological examination - LARVOSCOPY and methods?
Larvoscopy: Principle of larvoscopy is active migration of larvae into a warmer and wetter environment. Used for detection of lung worms.
We create artificial condition for worms, we cannot wait too long for them to move, only few minutes. Do not use Formaline/alcohol as it kills the larvae.
Vajda method: for investigation of feces in small ru (more firm feces), rabbits, hares and wild ruminants.
3g of feces, put into net/metal sieve, on the watch glass/beaker.
Add tap water (40 degrees) up to 1/3 of the sample, and leave for 15-20 minutes. (make sure bottom of net touches water).
Examine sample under microscope (low power)
Drops of Lugol`s iodine will kill and fix larvae
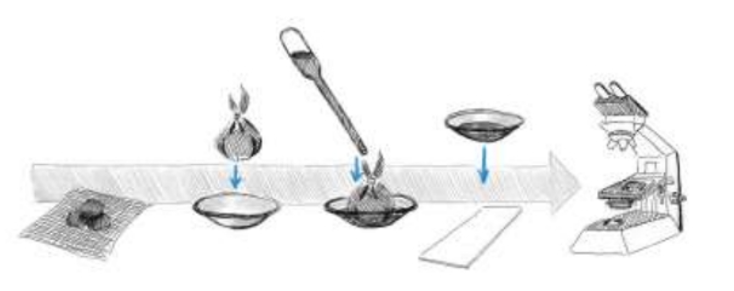
Baermann method – for investigation of cattle, horse and carnivore feces (no formed feces)
Weight 10-20g of feces and break them up slightly and wrap in a double layer of gauze
Place it in Baermann apparatus (a plastic funnel with a rubber tube attached to its end and closed with a clip)
Add water (40 degrees) until feces are covered
Leave overnight for 16-20 hours at room temperature
Collect the liquid content from the end of the rubber tube and place it on the watch glass, examine under microscope
Drops of Lugol`s iodine will kill and fix larva
1a) Ruminants species List - For question - coprological examination of ruminants, flotation methods, and Larvoscopy.
Species List:
Protozoa:
both: cryptosporidium parvum (SI), trichomonas foetus B (reproductive organs), Giardia duodenalis/bovis.
small ru: Eimeria (Parva, ovina, intricata) - SI
Large ru: Eimeria (Zuernii, bovis) - SI
Trematoda:
Fasciola hepatica, gigantica (liver, bile duct)
Fascioloides magna (liver)
Paramphistomum cervi (rumen)
Dicrocoelium dendriticum (bile duct, pancreatic duct, gall, bladder, liver)
Cestoda:
Small ru: Moniezia expansa
Large ru: Moniezia benedeni
Nematodes:
small ru:
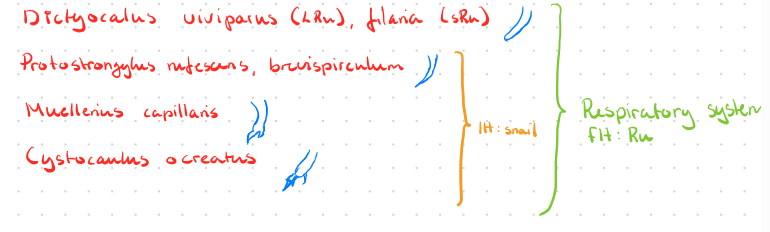
lungs: Dictyocaulus filaria, Muellerius capillaris, cystocaulus ocreatus, Protostrongylus rufescens.
Abomasum: Marshallagia marshalli, Trichostrongylus axei (also in SI), Ostertagia circumcinta, Haemonchus contortus.
SI: Strongyloides papillosus, capillaria bovis, Nematodirus spathiger, cooperia curticei.
LI: Oesophagostomum venulosum, trichuris ovis
Large Ru:
Lungs: Dictyocaulus viviparus
Abomasum: T. axei, ostertagia ostergi, M. marshalli, haemonchus placei
SI: S. papillosus, N. helveticus, toxocara vitulorum (calf), capillaria bovis, cooperia oncopheria.
LI: trichuris globulosa, Oesophagostomum radiatum, bunostomum phlebotomum.
Ectoparasites:
Ixodes ricinus - hard tick
Sarcoptes scabi - burrowing mite
Hypoderma - flies
bovis, lineatum - large ru
diana - small
Dalmalina - chewing lice
bovis - large
caprae, ovis - small
Linognatus - sucking lice
vituli - large
ovitus -small
Oestrus ovis (flies), psoroptes ovis (non burrowing mite) - small ru
Haematopinus eurysternus (sucking lice), demodex bovis (burrowing mite), chorioptes bovis (non-burrowing) - large ru
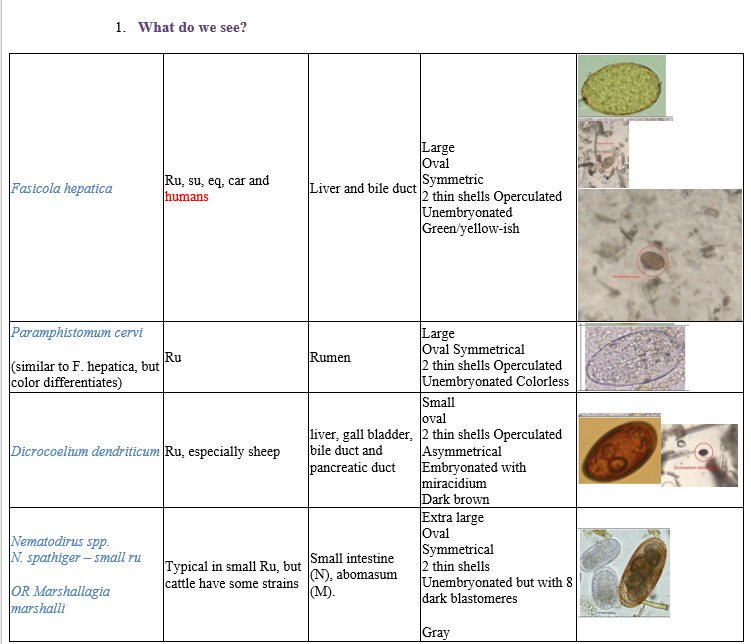
1b) Coprological examination of ruminants – flotation methods.
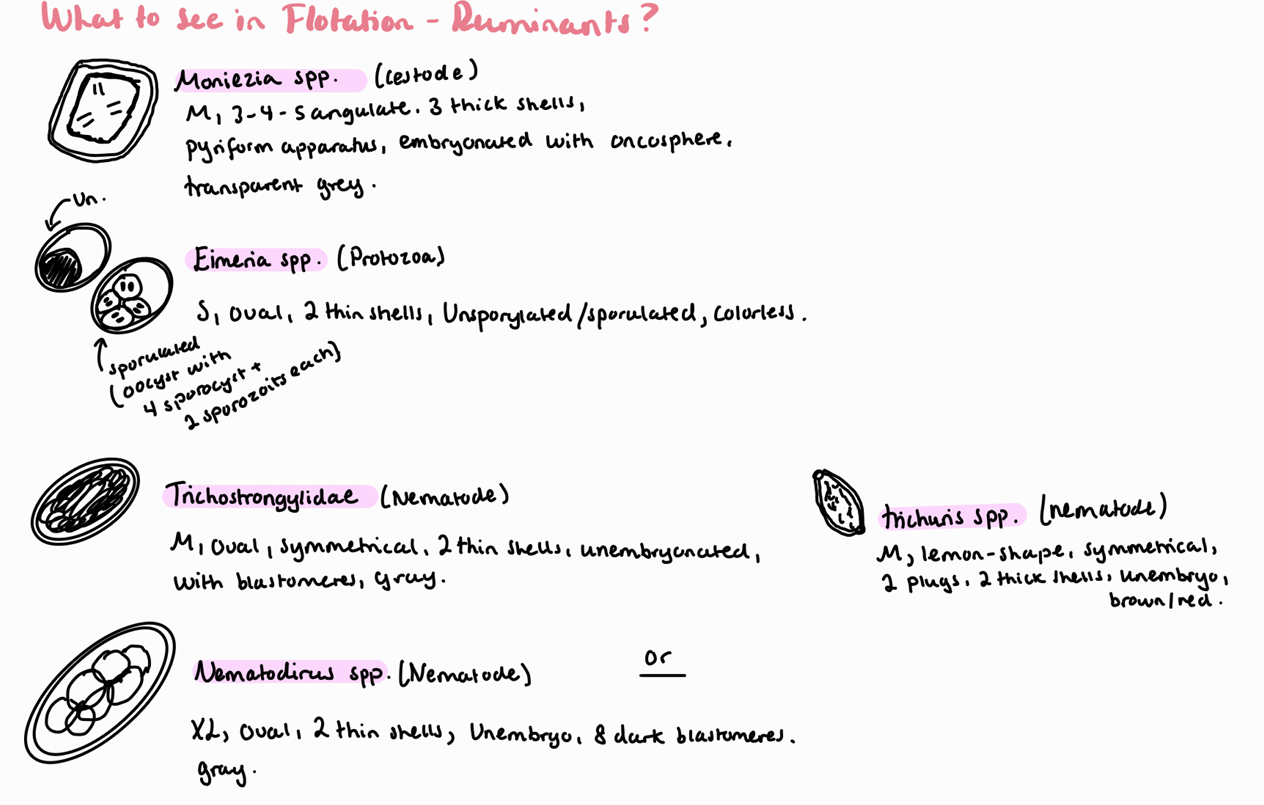

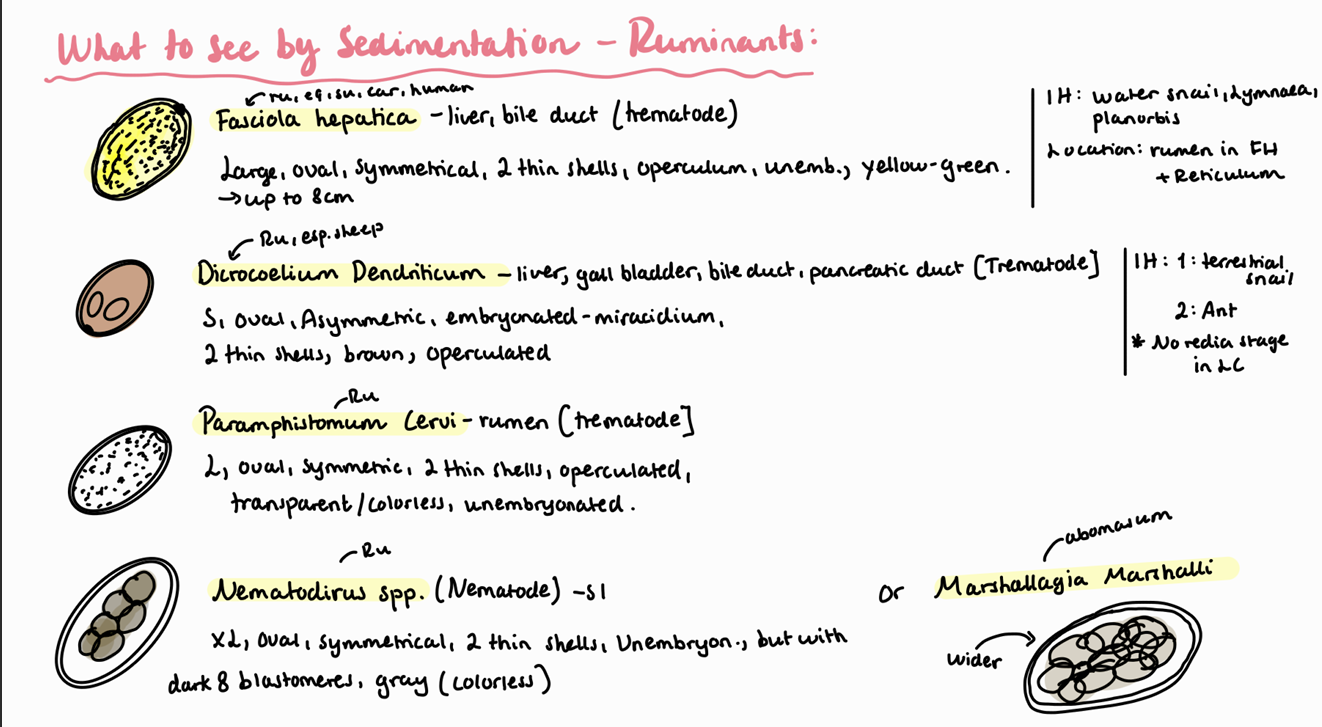
Coprological examination of ruminants – sedimentation methods.
Notes:
F. hepatica is up to 8 cm. IH: water snail, Lymnaea
Paramphistomum is up to 12 cm. IH: water snail, lymnaea, Planorbis. Located in the rumen of FH and reticulum.
D. dendriticum is 6-12 mm long. IH1: terrestrial snail and IH2: ant. Location: liver/bile duct of ru, these have no redia stage in LC.
Coprological examination of ruminants – larvoscopy.
Lung worms of ruminants are: Muellerius Capillaris, Dictyocaulus Filariae (viviparous in cattle), protostrongylus rufescens, Cystocaulus Ocreatus, Neostrongylus Linearis.
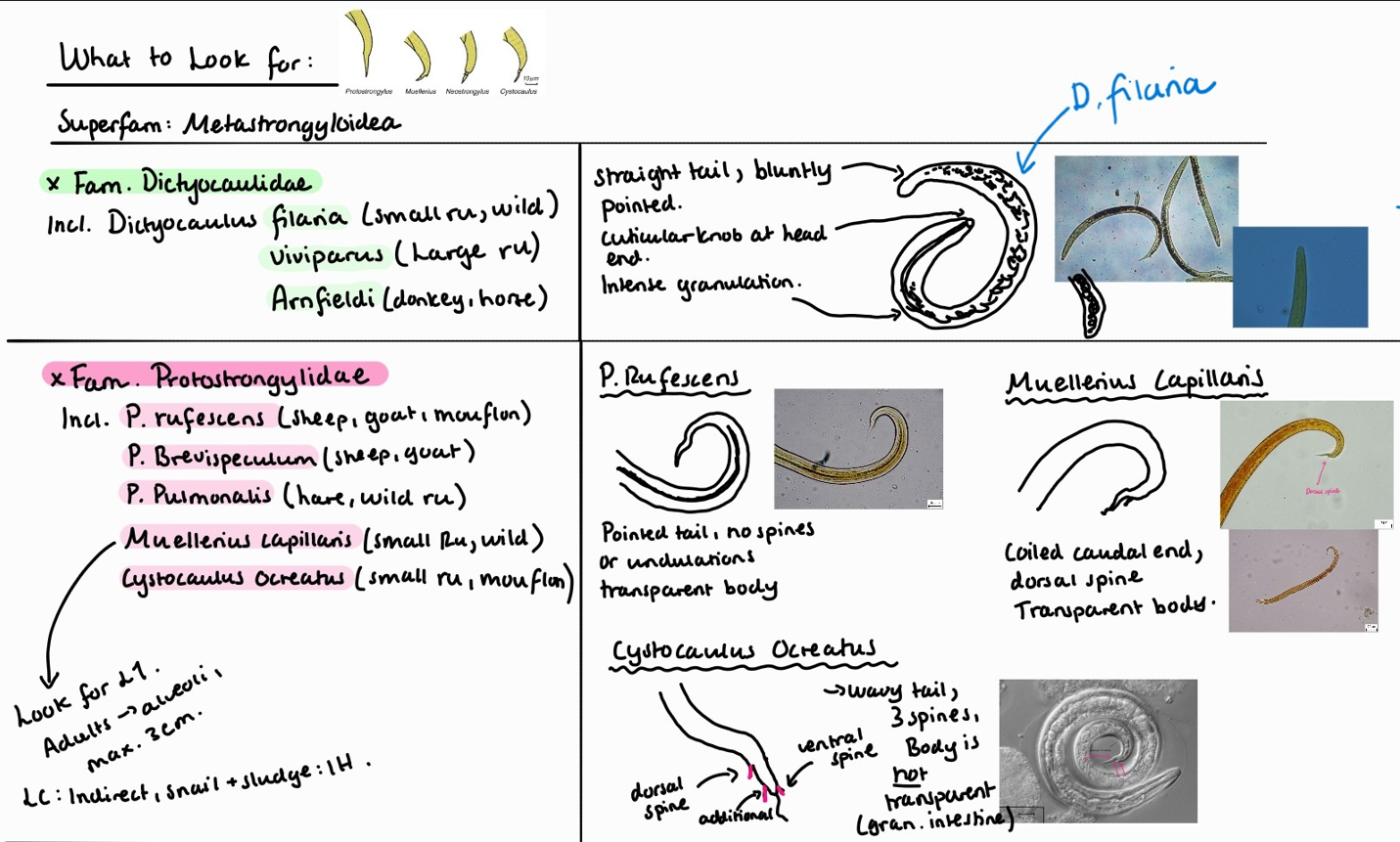
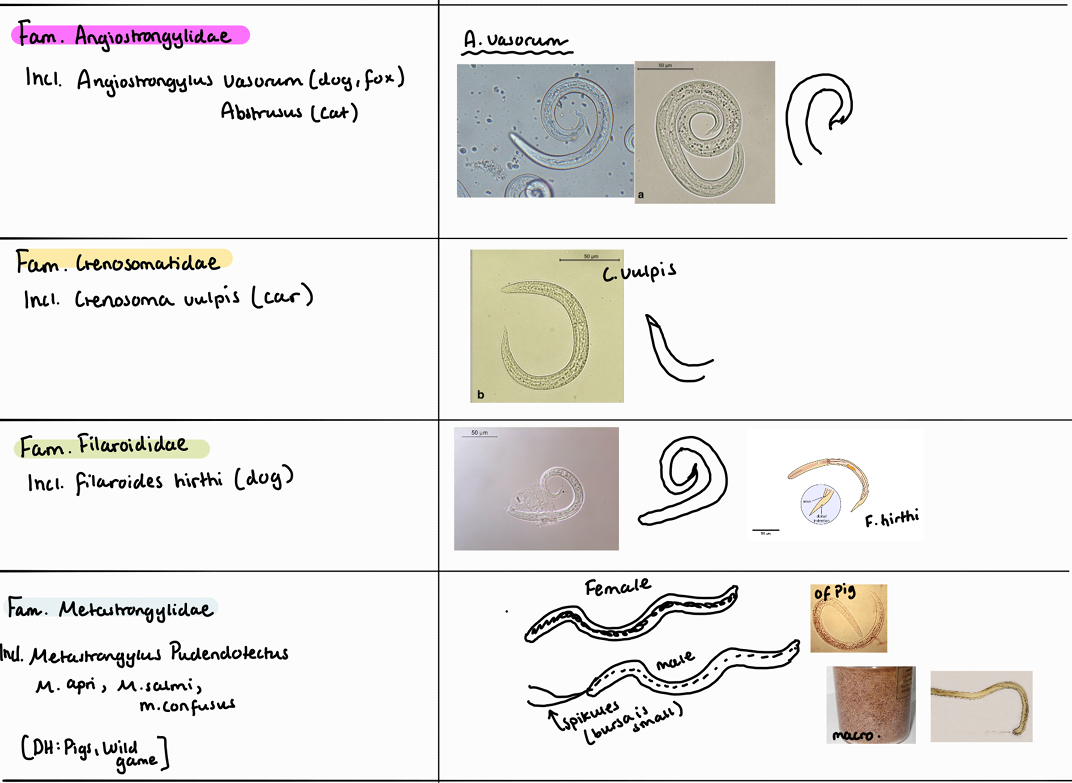
In Muellerius capillaris, we look for L1.
Adult worms are found in alveoli.
They are max 3cm.
Life cycle is indirect with snail and sludge as IH.
Note: look at the end of the tail, it has a hook! Posterior end of male adult is coiled.
4a. Species list to horses - question.
Protozoa:
Eimeria Leuckarti - SI (wont get under microscope)
Cryptosporidium Parvum - rare
Trematoda:
F. hepatica (liver) - rare
Dicrocoelium dendriticum (liver)
Schistosoma (japonicum, nasalis, spindale - occassionally)
Cestoda:
Anoplocephala (magna, perfoliata) - SI
Paranoplocephala mamillana - SI
Nematoda:
lungs: dictyocaulus arnfieldi (both in flotation + larvoscopy)
stomach: trichostrongylus axei
SI: parascaris equorum, strongyloides westeri
LI:
large strongylus: vulgaris, edentatus, equinus
small strongylus: cyathostomum, cylicocyclus
oxyuris equi
Ectoparasites:
Ixodes ricinus - hard tick
haemophysialis spp. - hard tick
dermacentor spp. hard tick
demodex equi
dalmalina equi (norway) - chewing lice
haematopinus asini - sucking lice
and: brachycera (flies), rhinoestrus - purpureus, usbekistanicus (flies), gasterophilus - intestinalis, pecorum (flies)
4b. Coprological examination of horses.
Flotation method according to Breza of horse feces samples - findings are:
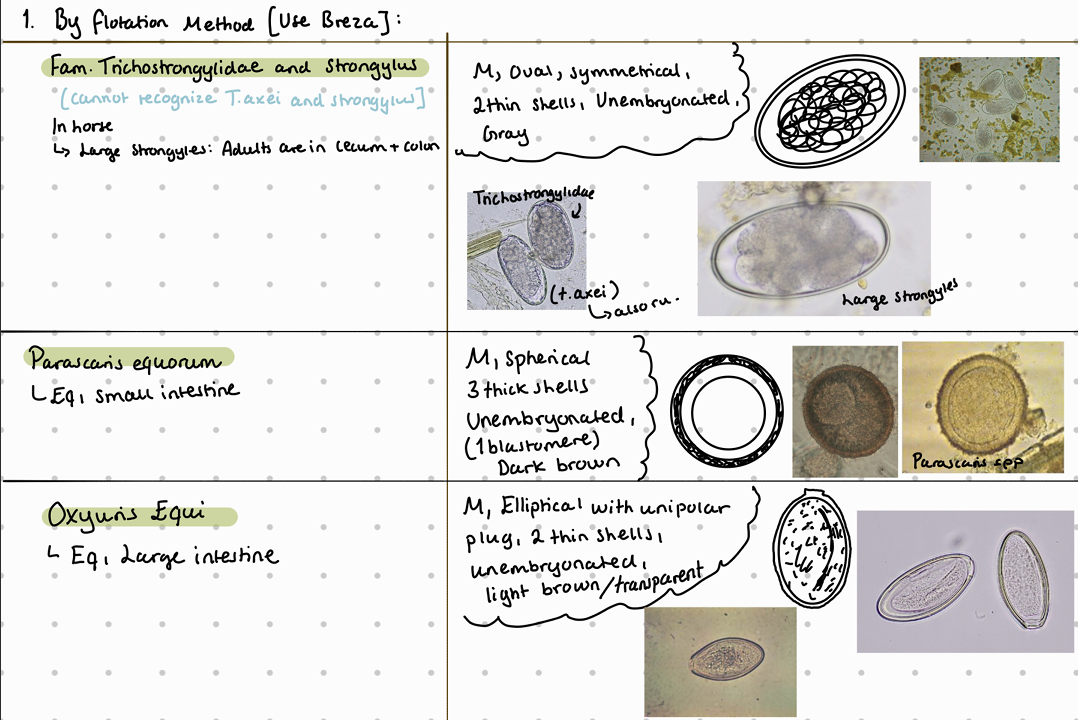
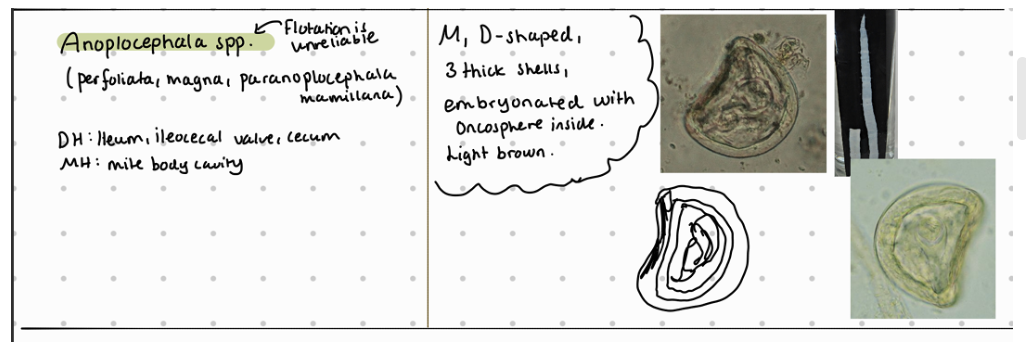

Eggs of family anoplocephalidae are similar (also to Ru anoplocephalia worms) - they all have irregular shape like D or box shaped.
If we do sedimentation method for horses, we might find:
F. hepatica, D. dendriticum
Tidligere: P. equorum, strongylus spp. qs:
Infective stage of parascaris, migration: L3, larva within egg. Migration: after horse eats egg with L3 → hatches in SI → goes through intestinal wall → migrate via veins to the liver (where it molts to L4) → 2 weeks → migrates to heart and lungs → travels to bronchi → trachea, coughed up and then swallowed → mature to L5 and then adults in SI.
Migration of strongylus vulgaris, equinus, edentatus
S. vulgaris: L3 larvae penetrates the intestinal mucosa → L4 in the submucosa → arteries and migreate to cranial mesenteric artery → molt to L5 → migrate to intestinal wall, forming nodules in cecum and colon → adults.
S. equinus: L3 → wall of SI, cecum, colon → encapsulating in nodules → L4 leaves and goes to liver → migrates back to LI → molting to L5 during this return.
S. edentatus: L3 excyst in the SI → gut wall → molts to L4 → migrate to root of mesenteric artery → liver, lungs → returns to cecum wall and right ventral colon.
IH of anoplocephala: Oribatid mite
metacestode in anoplocephala: Cysticercoid (develops within the oribatid mite).
5a) List of species for Pig - to qs. coprological examination of pigs.
Species List:
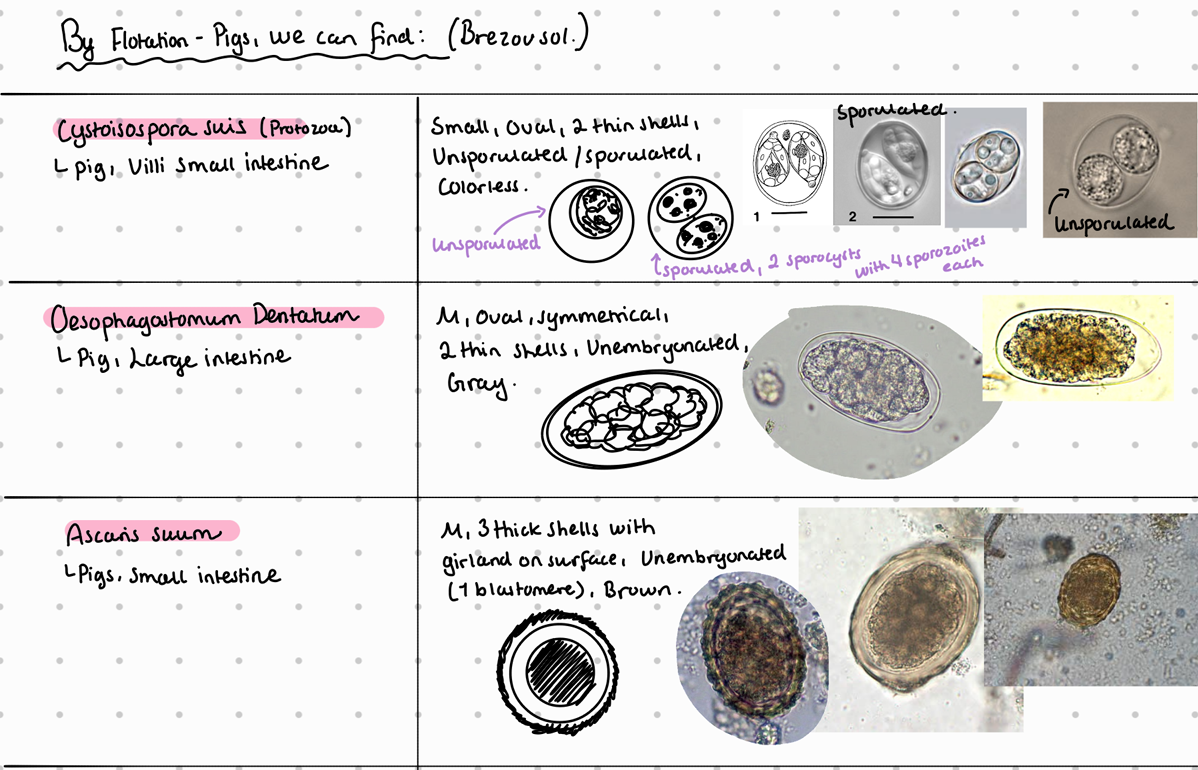
Protozoa:
Eimeria (Debliecki, Poliata, Spinosa, suis, scabra, neodebliecki)
Cystoisospora suis
Cryptosporidium suis/parvum
Trematoda:
Fasciola hepatica
Dicrocoelium Dendriticum (rare)
Fasciolopsis buski - liver
Schistosoma (japonicum, spindale, nasalis)
Cestoda:
Taenia solium (IH: pig, human), hydatigenea (SU, pig - cysticercus tenuicollis), multiceps (IH: sheep, cattle, goat, coenurus cerebralis)
Echinococcus granulosus (IH: sheep, human, forming hydatid cysts in liver, lung, brain and bone marrow)
Nematoda:
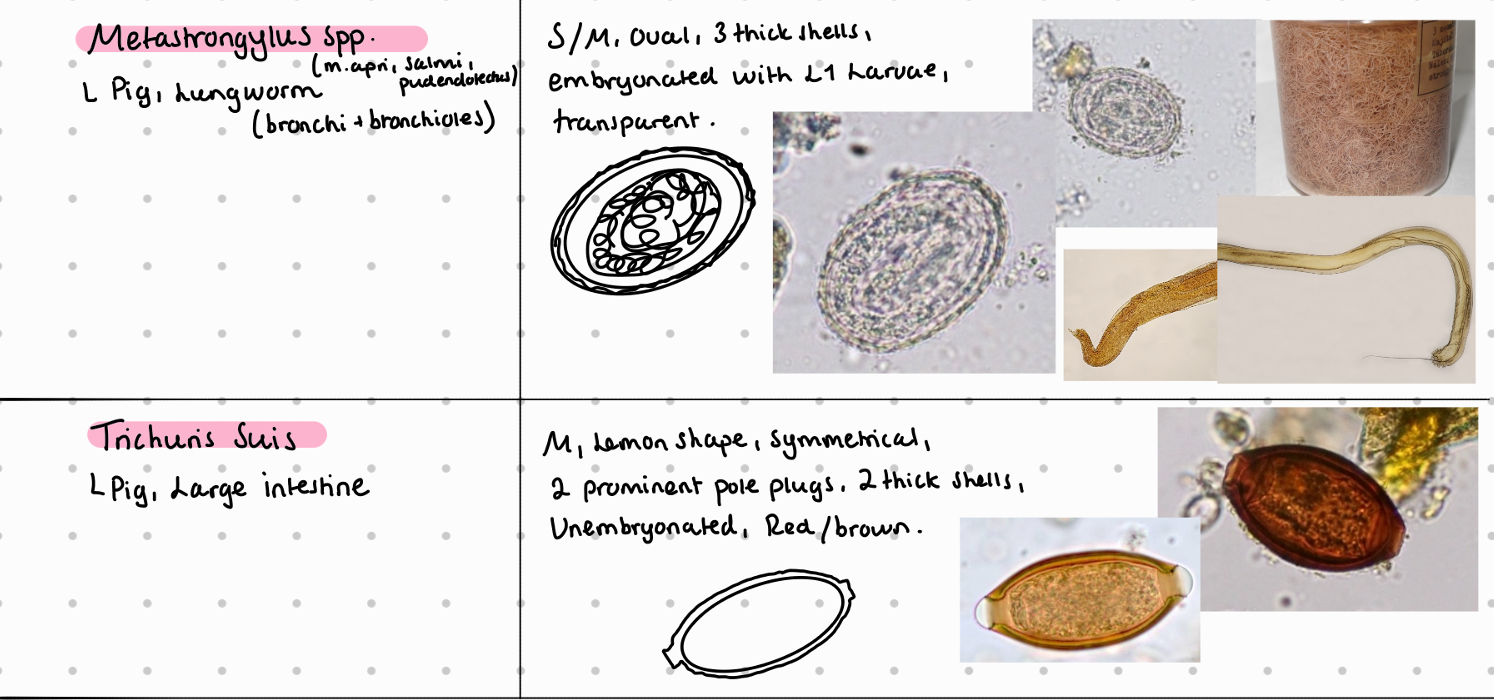
Lung: Metastrongylus (Apri, salmi, pudendotectus)
Kidney: stephanurus dentatum
Stomach: hyostrongylus rubidus
SI: strongyloides ransomi , ascaris suum
LI: Oesophagostomum dentatum, trichuris suis
Acanthocephala:
Macracanthorhynchus hyradinaceus - SI
Ectoparasites:
Demodex phylloides
sarcoptes suis/scabei
Haematopinus suis
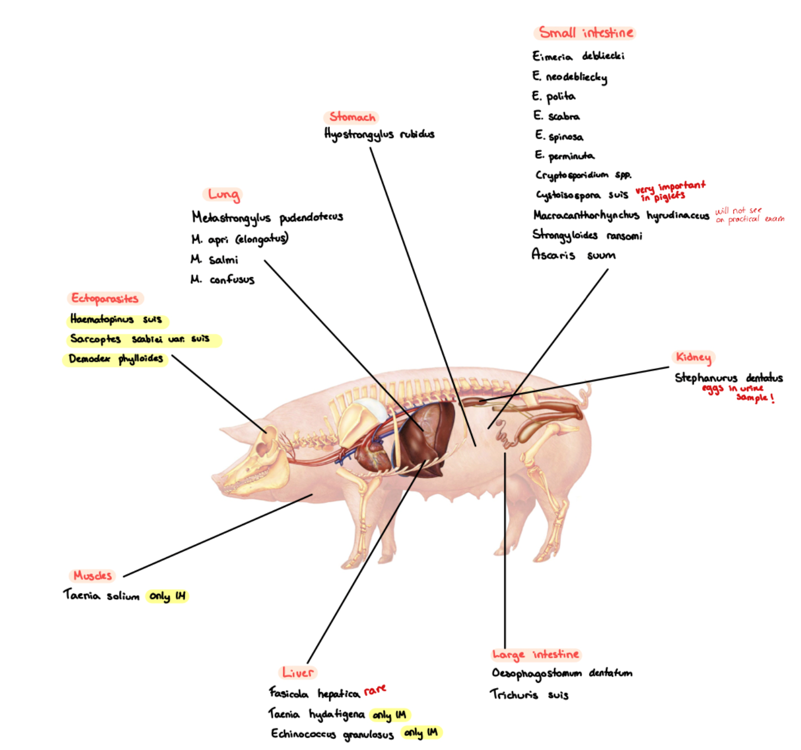
5b) Coprological examination of pigs.
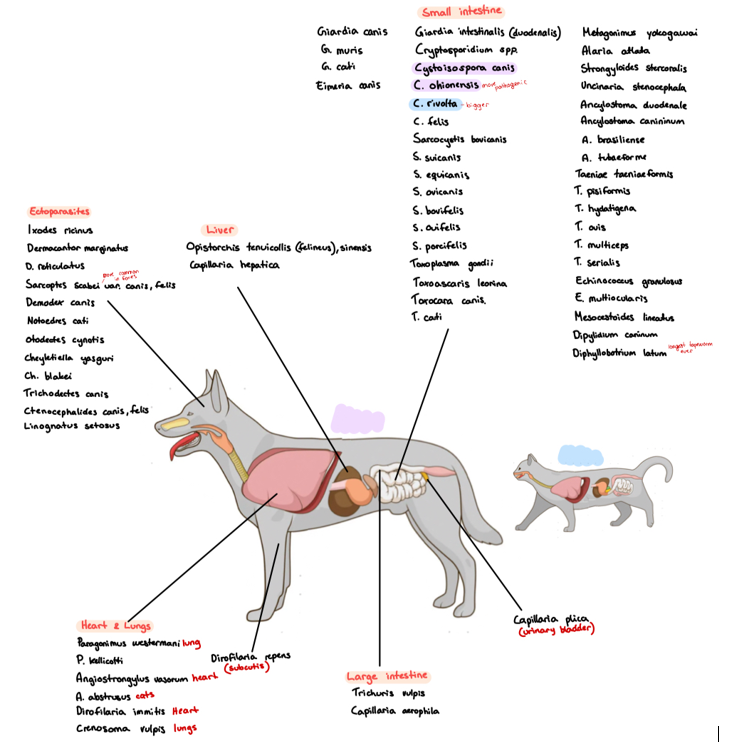
6a) Species list for carnivores - qs. Coprological examination of carnivores.
Species list:
Protozoa:
Eimeria canis, cati (rare)
Giardia (duodenalis/intestinalis, canis, cati)
Cryptosporidium (canis, felis)
Cystoisospora (canis, felis, revolta)
Sarcocystis (bovicanis, bovifelis)
Toxoplasma gondii
Neospora caninum
Babesia (canis, felis)
Trematoda:
Lung: Paragonimus (westermani, kellicotti)
bile ducts: opistorchis (tenuicollis, felineus, sinensis)
SI: Alaria (alata, canis), Metagonimus yokogawai, Heterophyes heterophyes, echinochasmus perfoliatus (intestine)
Mesenteric vessels: Schistosoma japonicum
Cestoda: small intestine
Taenia
serialis (coenurus serialis)
Multiceps (coenurus cerebralis)
pisiformis (cysticercus pisiformis)
Hydatigenea (cysticercus tenuicollis)
ovis (cysticercus ovis)
taeniaformis (cysticercus fasciolaris)
cervi (cysticercus cervi)
mesocestoides lineatus
Echinococcus (granulosa, multilocularis)
diphyllobothrium latum
dipylidium caninum
Nematoda:
Lungs: crenosoma vulpis, capillaria aerophile,
C. plica, C. hepatica.
Heart: Dirofilaria (immitis, repens), Angiostrongylus (abstrusus, vasorum)
SI: Ancylostoma (duodenum, caninum), Toxocara (canis, cati), toxascaris leonina, strongyloides stercoralis
LI: trichuris vulpis
Ectoparasites:
Ixodes ricinus - hard tick
dermacentor (marginatus, reticulatus) - hard tick
demodex canis - burrowing mite
sarcoptes scabei canis/cati - burrowing mite
ctenocephalides (canis, felis) - flea
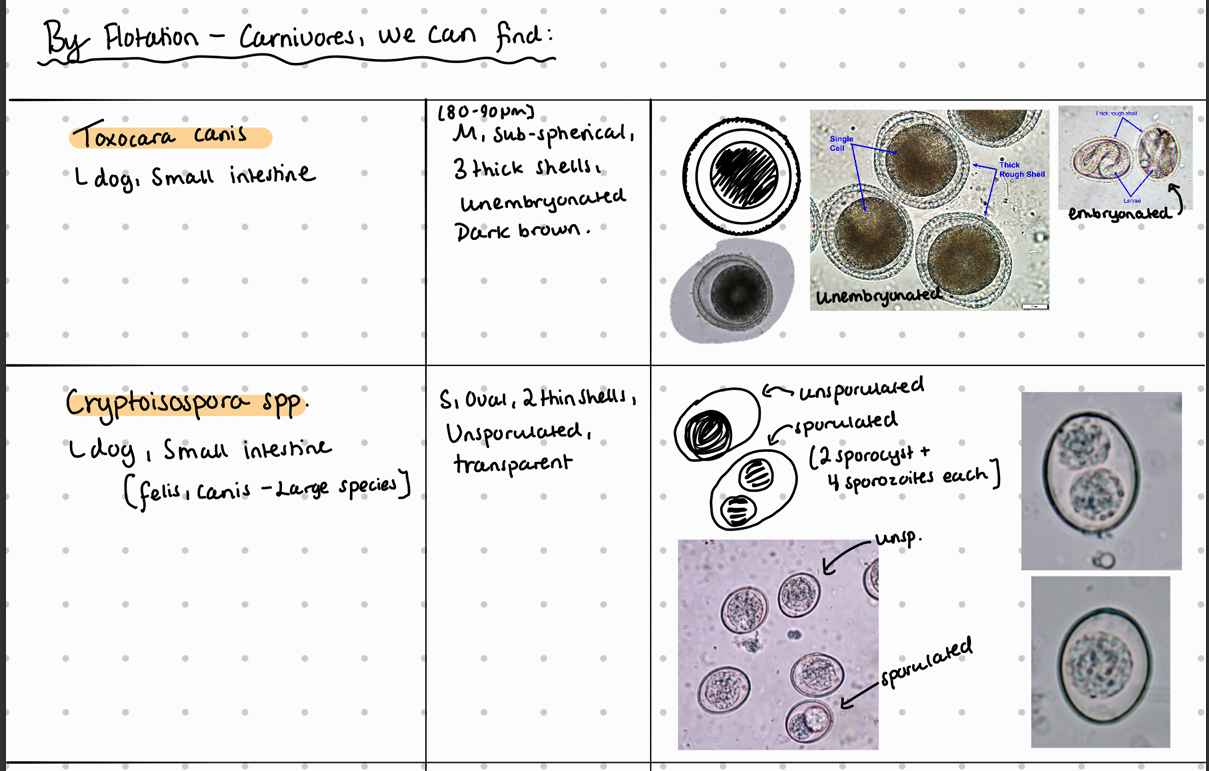
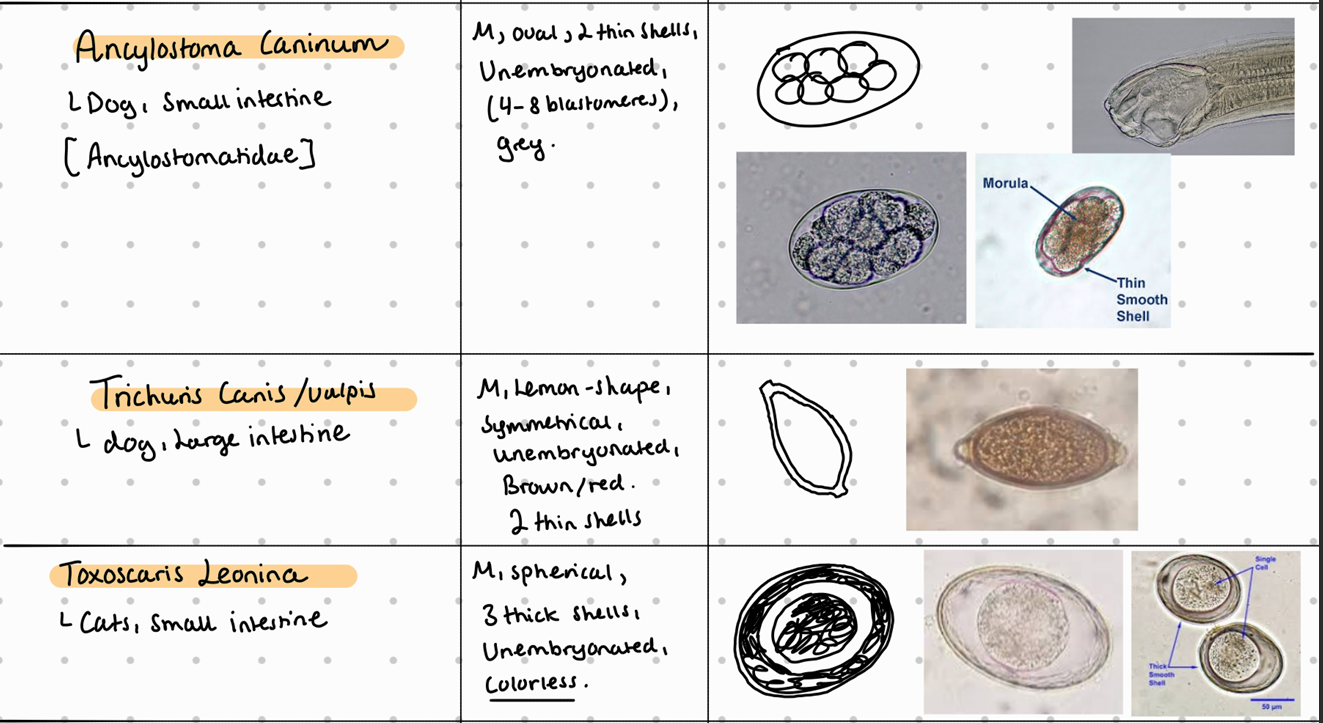
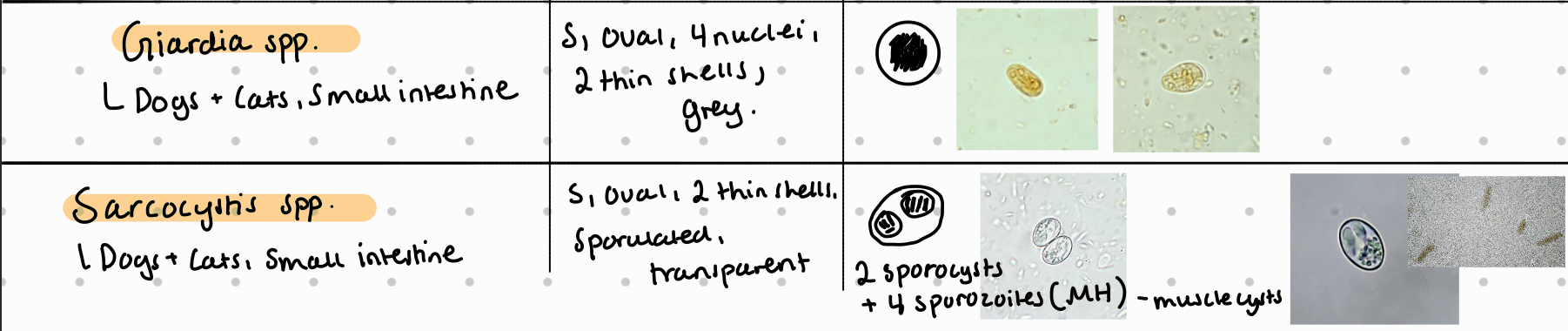
6b) Coprological examination of carnivorous.
7a) Species list for Poultry - for qs. coprological examination of poultry
Species List:
Protozoa:
Trichomonas gallinae (tetratrichomonas ansertis, gallinarum, anatis) - SI
Cryptosporidium (bailey, meleagridis (turkey), galli) - SI
Eimeria
Chickens: Maxima (jejunum), necatrix (jejunum), mitis (jejunum), tenella (cecum), brunetti (ileum, cecum, rectum)
Turkey: meleagridia
Geese/duck: truncata
Histomonas meleagridis (cecum)
Trematoda:
Prosthogonimus (ovatus, cutaneus) - Bursa fabricii, oviduct
Echinostoma revoltum - LI, rectum
Cestoda:
Diphyllobothrium (dendriticum, latum)
hymenolepsis (lanceolata, carioca)
Ligula intestinalis
Raillietina (ransomi, tetragona)
Choanataenia infundibulum
Nematoda:
syngamus trachea
Ascaridia (galli, columbae, dissimlis) - SI
Trichostrongylus tenuis - SI
Heterakis (gallinarum, dispar) - LI
Capillaria spp.
Acanthocephala: filicolis anatis, Polymorphius (magnus, minitus)
Ectoparasites:
Argas - soft tick
reflexus (pigeon)
persicus (chicken)
Cnemidocoptes (mutans, gallinae) - chewing mite
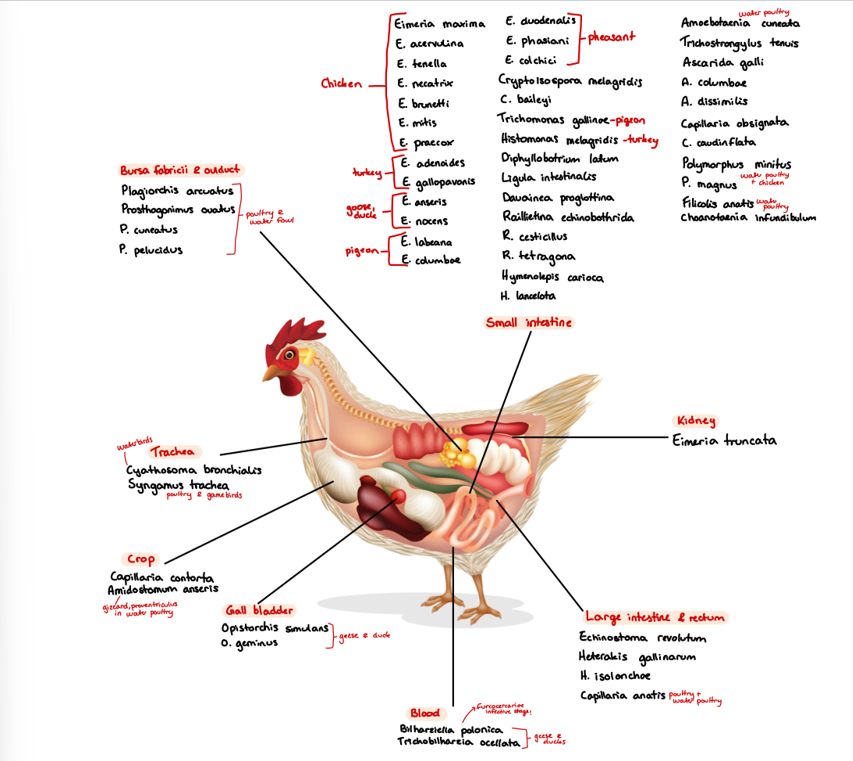
7b) Coprological examination of poultry
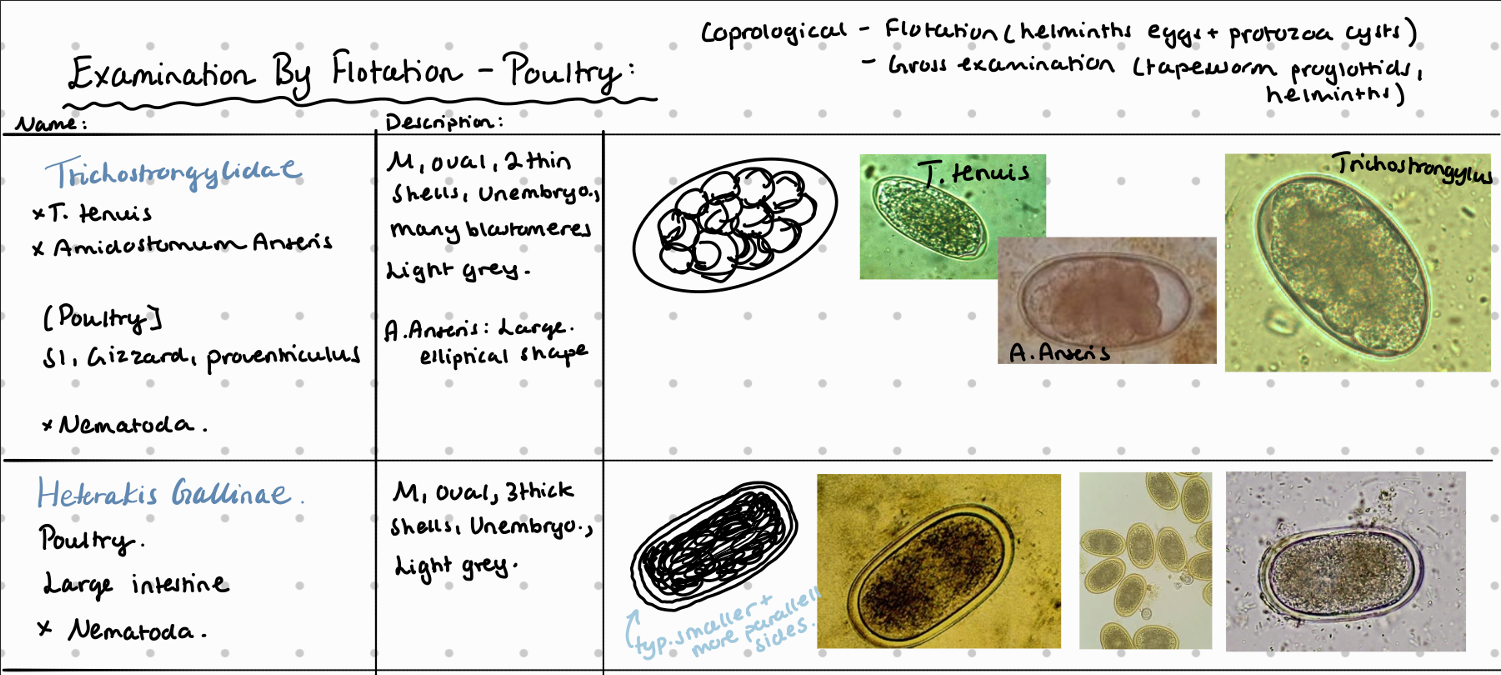
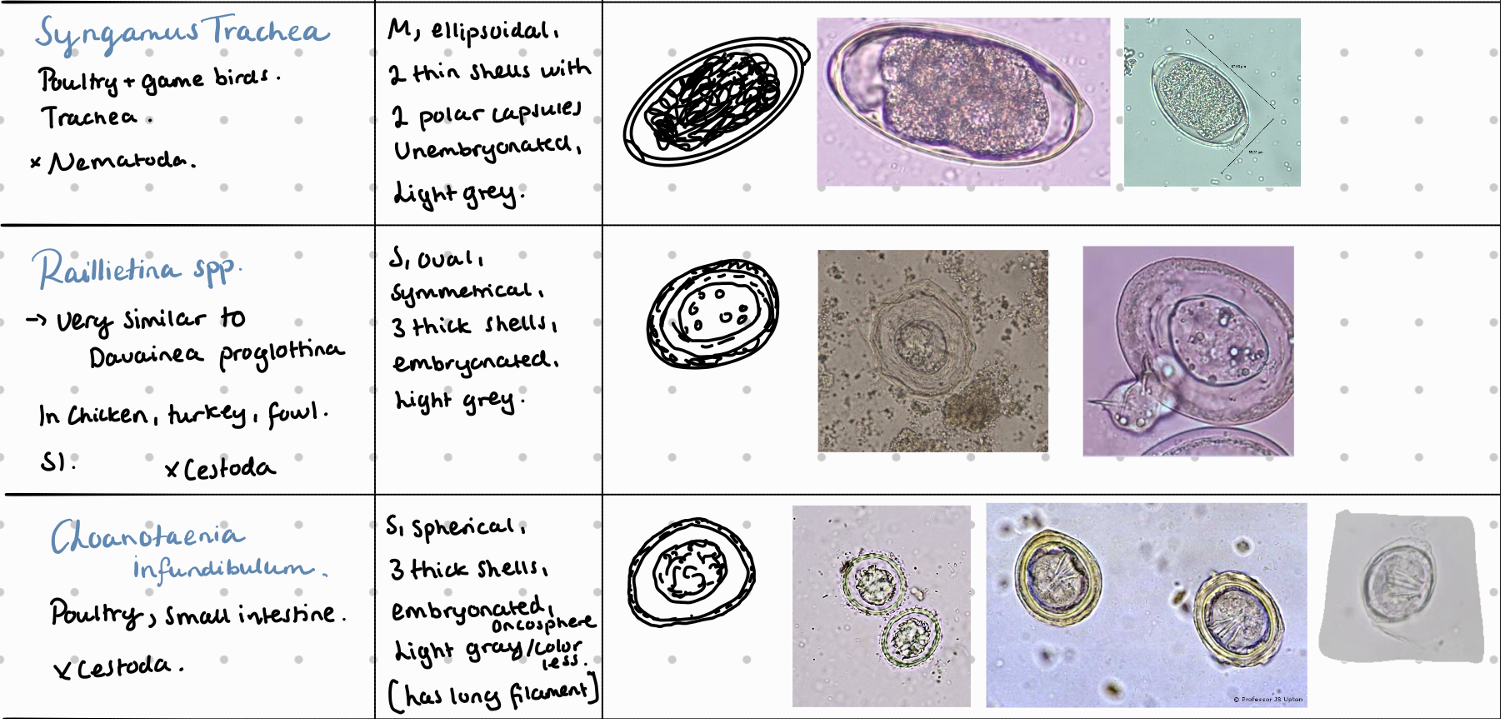
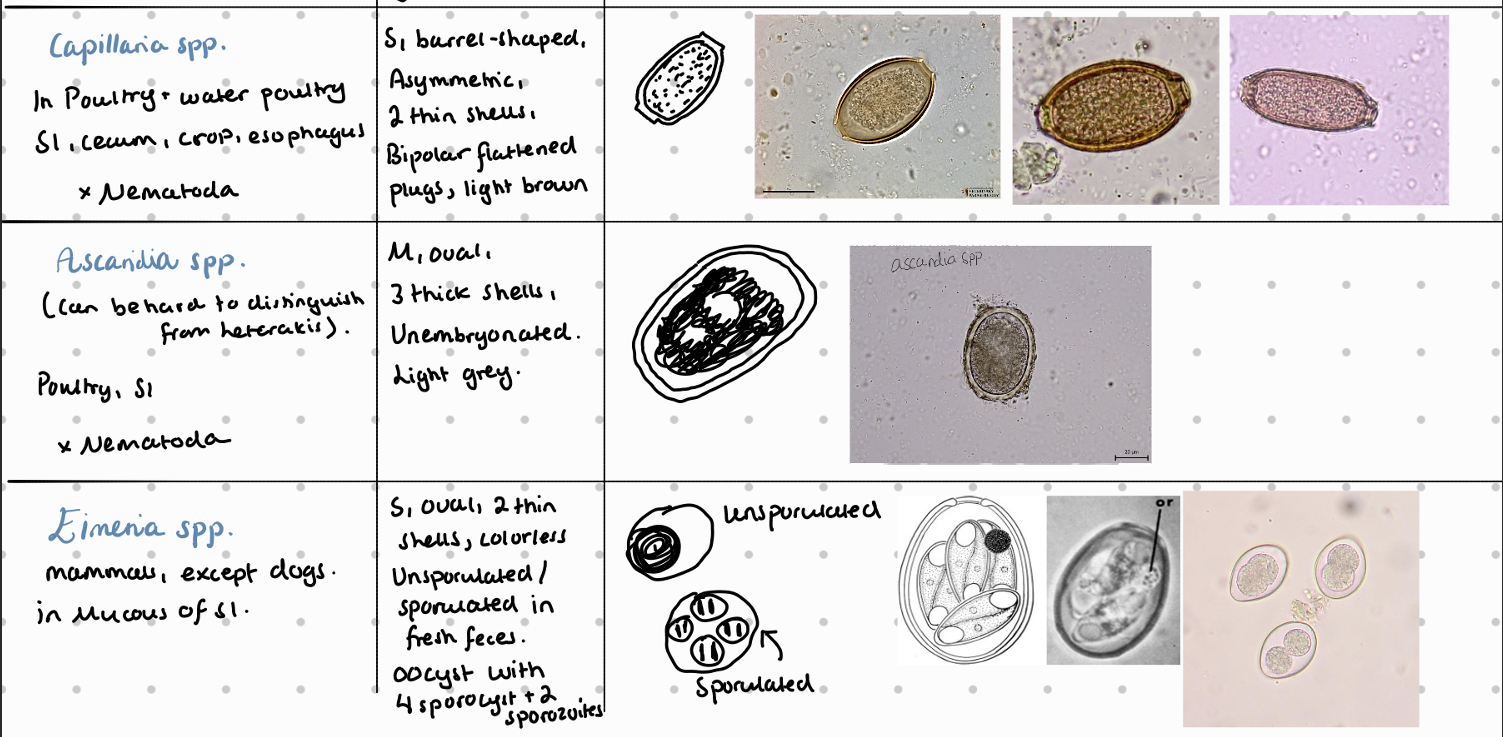
8a) Species list of rabbits/hares to qs - coprological examination of rabbits and hares
Species list:
Protozoa:
Eimeria (SI)
Europea (hare), intestinalis, piriformis, magna, Stiedai (liver), Hungerica, leporis
Encephalitozoon cuniculi (parasitic fungi)
Also: Entamoebe cuniculi, sarcocystis cyniculi, toxoplasma gandii (can act as IH)
Trematoda:
F. hepatica
D. dendriticum
Schistosoma japonicum (blood)
Cestoda (SI):
Cittotaenia (ctenoides, pectinata, denticulate, variabilis)
Taenia/coenurus serialis - can be IH
Andrya cuniculi
Nematoda:
Lungs: Protostrongylus pulmonis
SI: strongyloides papillosus, trichostrongylus (retortaeformis - stomach, colubriformis)
LI: passalurus Ambigus, trichuris leporis
Stomach: Graphidium strigosum
Ectoparasites:
Psoroptes Cuniculi - non-burrowing mite
also: Cheyletiella parasitovorax - non-burrowing mite
Otobius lagophilus - soft tick
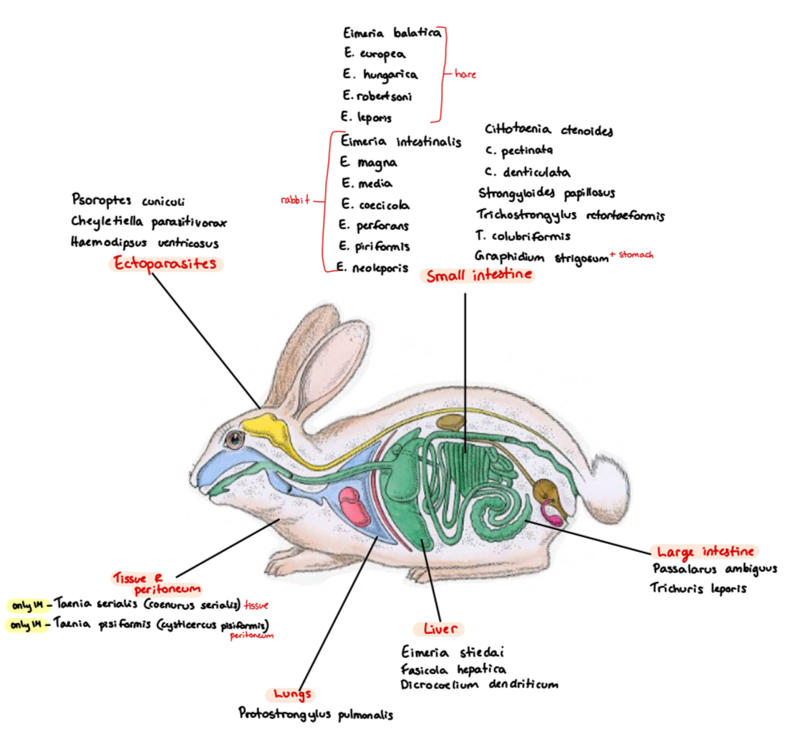
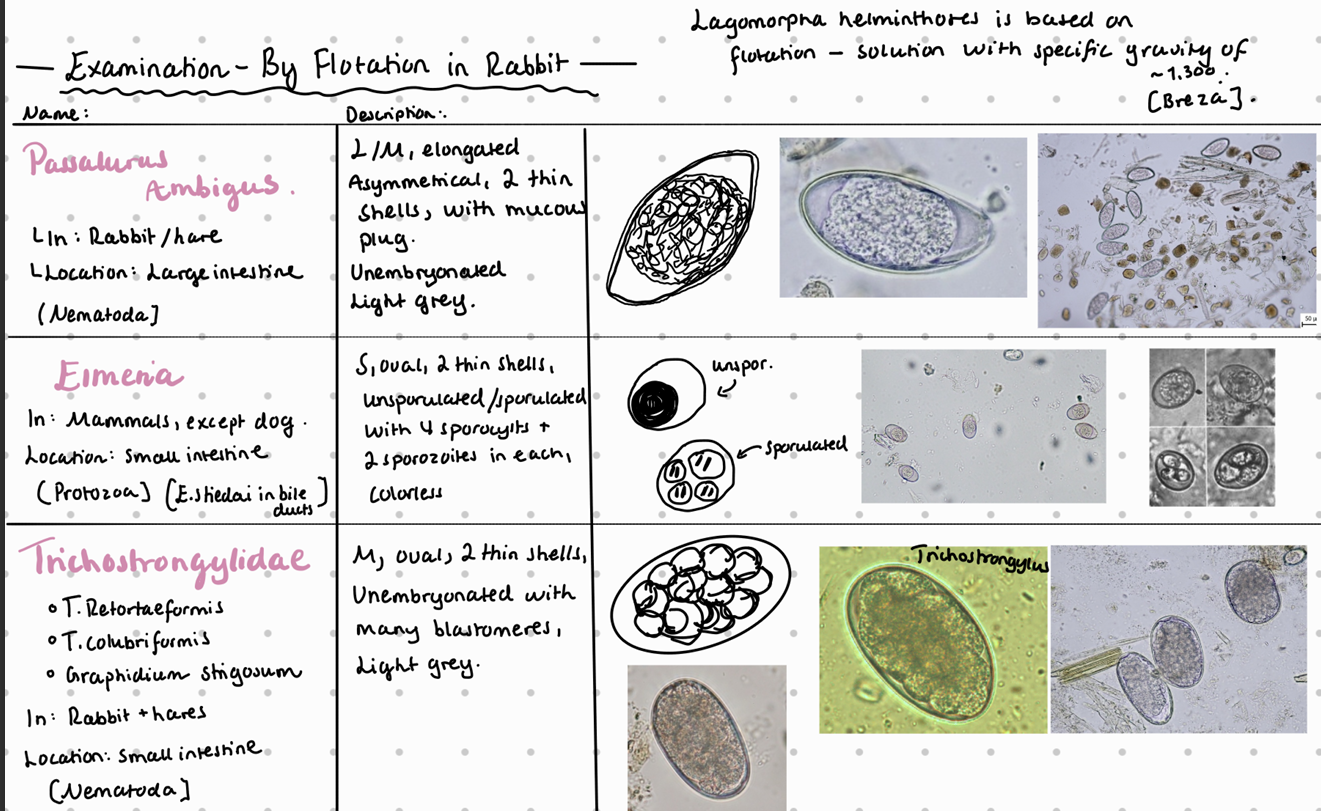
8b) Coprological examination of rabbits and hares.

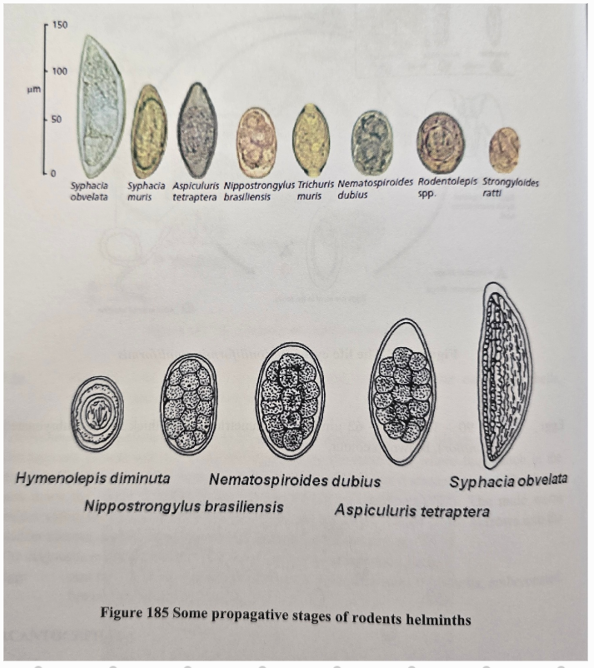
9a) Species list to Rodents - for qs. coprological examination of rodents
Species list:
Protozoa:
Eimeria (falciformis - mouse, myarii, ratti - rat) - SI
Cryptosporidium (muris - mouse, Parvum - hamster) - SI
Giardia (muris, microti)
Cystoisospora rati, trichomonas muri
Trematoda (liver):
F. hepatica
D. dendriticum
Cestoda:
Hymenolepsis (nana, diminuta, microstoma) - SI
Echinococcus Multilocularis
Nematoda:
Strongyloides Ratti - SI
Capillaria hepatica - liver
LI: aspiculuris Tetraptera (pinworm), syphacia muris, obvelata (pinworm), heterakis spumosa, trichuris muris
Acanthocephala:
Monilloformis Monilloformis
Ectoparasites:
Ixodes ricinus - hard tick
Dermacentor spp. - hard tick
Polyplax (spinulosa, sinulosa) - lice
Sarcoptes scabiei
flea: Leptopsylla segnis, nasopsyllus segnis
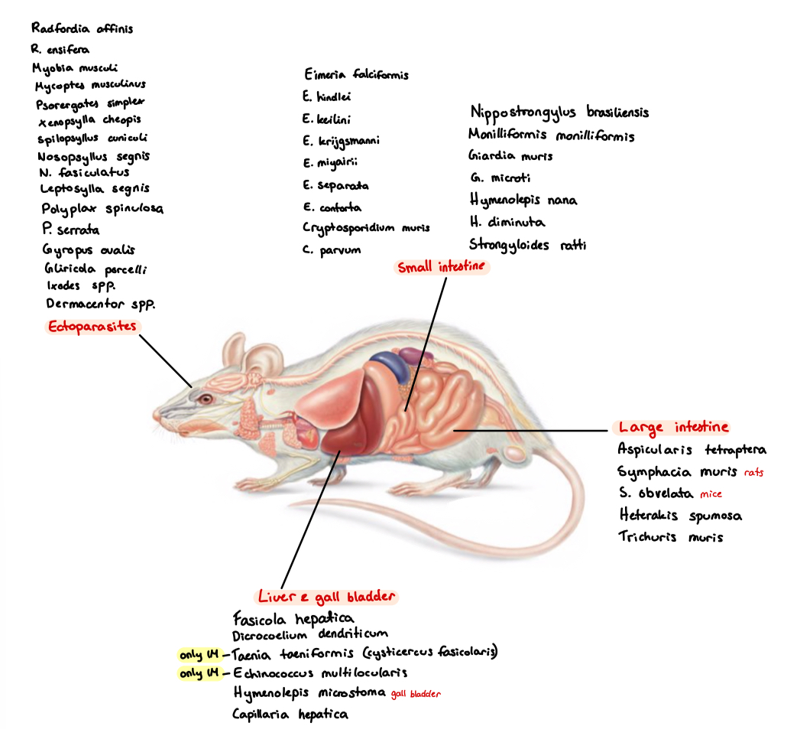
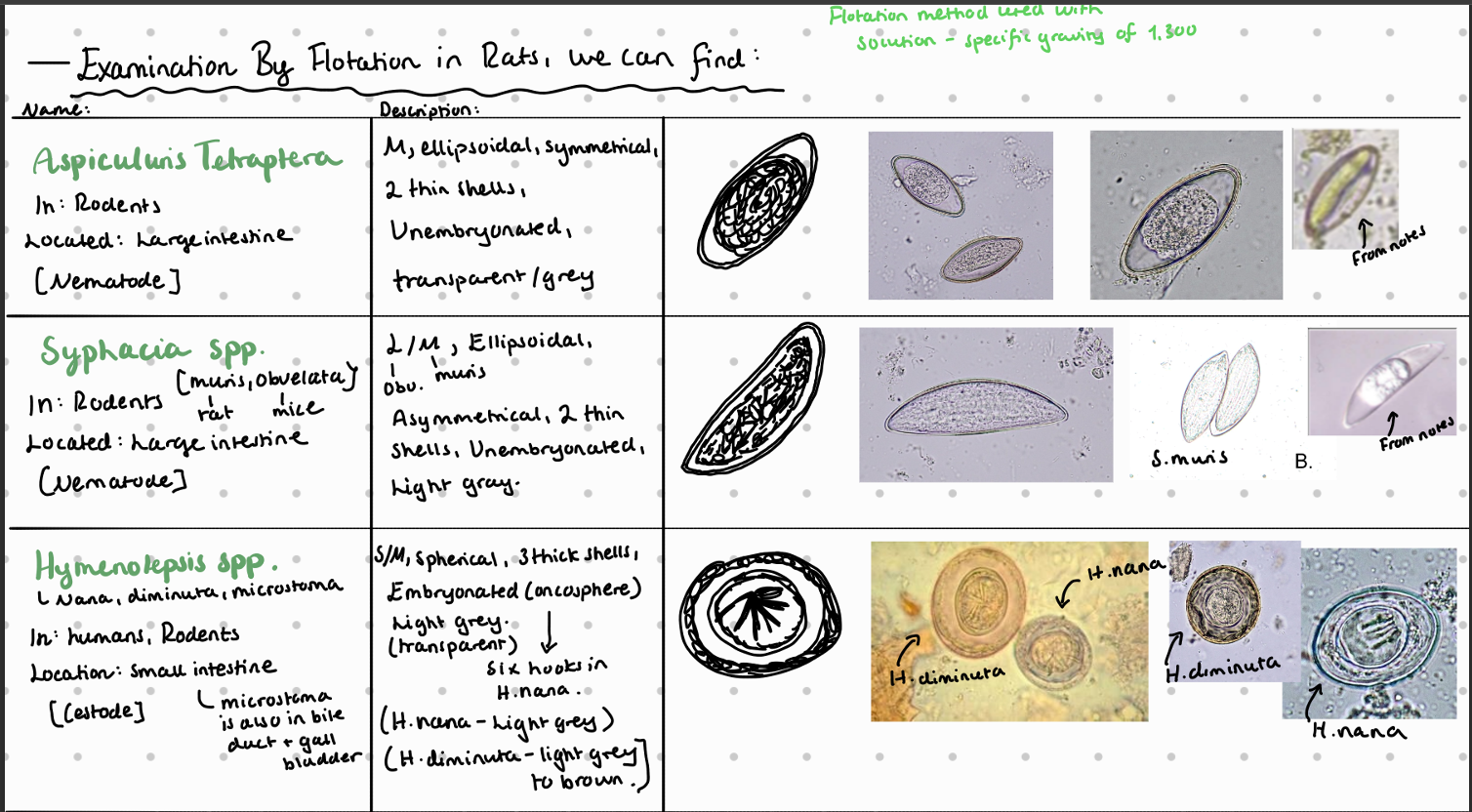
9b) Coprological examination of rodents.
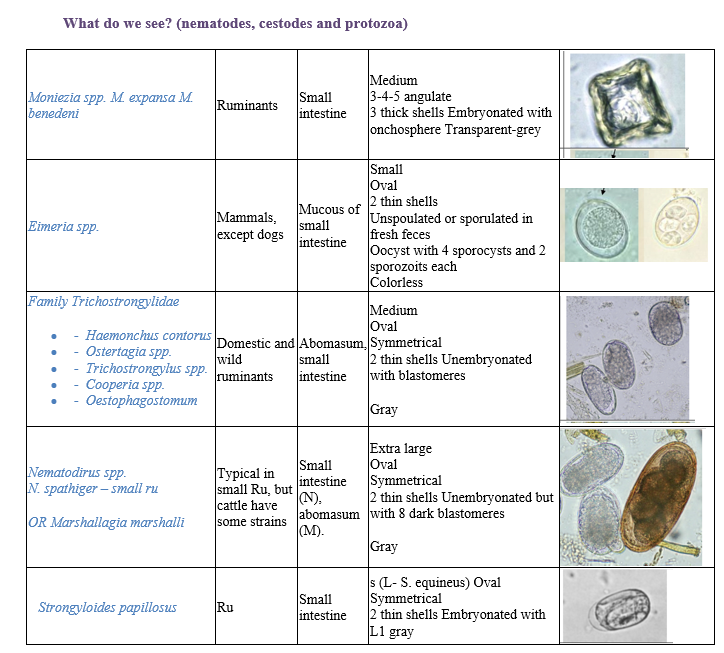
Fecal flotation method – What do we see?

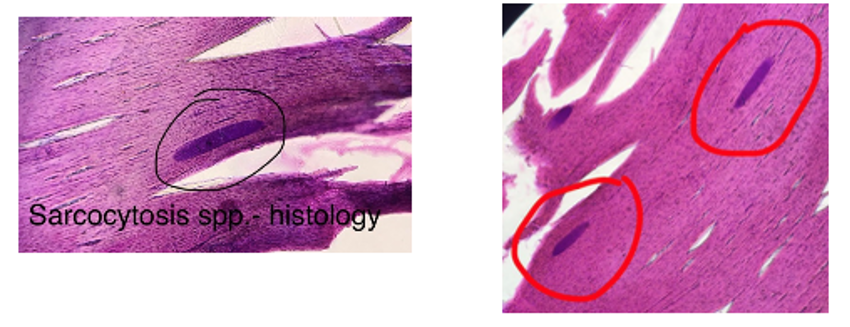
Diagnostic methods of sarcocystosis in IH.
Life cycle of Sarcocystis:
Heteroxenous: Requires both intermediate (IH) and final hosts (FH).
IH: Ingests sporocysts from FH feces → cysts excyst in IH intestine → merogony (3 generations) in blood vessel cells → merozoites become metrocysts → transfer to muscles via lymphocytes → become bradyzoites (infective to FH).
FH: Ingests cysts from IH muscles → gametogony occurs → bradyzoites in lamina propria of SI become macrogametes and microgametes → form zygote → oocyst develops → sporogony occurs inside FH → sporulated oocysts shed in feces.
Diagnosis
Fecal Flotation (FH): Detect 2 sporocysts, 4 sporozoites, S - size, oval, thin shells, embryonated, transparent.
Digestive Method (IH)
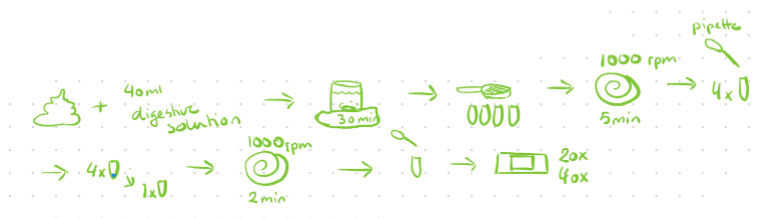
15g of meat sample is cut into small pieces and mixed with 40ml of digestion solution (trypsin + buffer) in electric mixer
Digestion of sample for 30 minutes
Filtrate sample through sieve into 4 tubes and centrifuge for 5 min at 1000 rpm
Supernatant is removed by pipette and sediment from 4 tubes is pooled into one tube, add water, then centrifuged again at 2 min for 1000 rpm
Supernatant is discarded and 2-3 drops from the sediment are placed on a glass slide, covered with a cover slip and examined, count macrocysts.
Other: Serology (ELISA), biopsy, histology, macroscopic detection (cysts in muscles) - sarcocystis gigantea/ovifelis of sheep in esophagus are known to have larger cysts. Or S. rileyi in chest muscles of ducts.
Cattle: Cysts often too small for detection during meat inspection.
Quantitative concentration methods
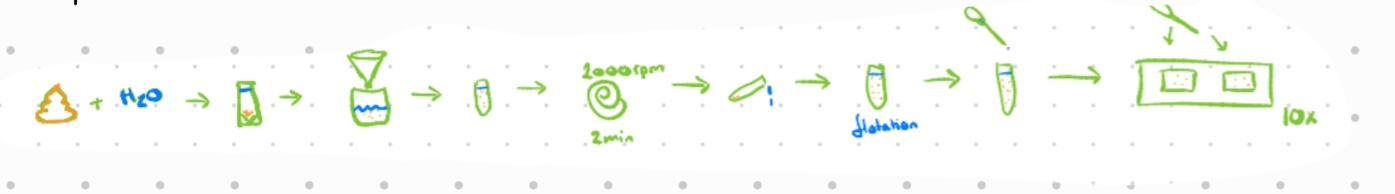
McMaster method:
Mix 3g of feces in 42ml of water and pour through a tea strainer/paper filter into centrifugation tube
Special beaker with 2 marks, one for water and one for feces
Centrifuge for 2 min at 2000 rpm
Decant (remove supernatant) and mix the sediment with flotation solution (1 cm from top)
With a pipette, transfer 1ml suspension to McMaster counting chamber and fill both chambers
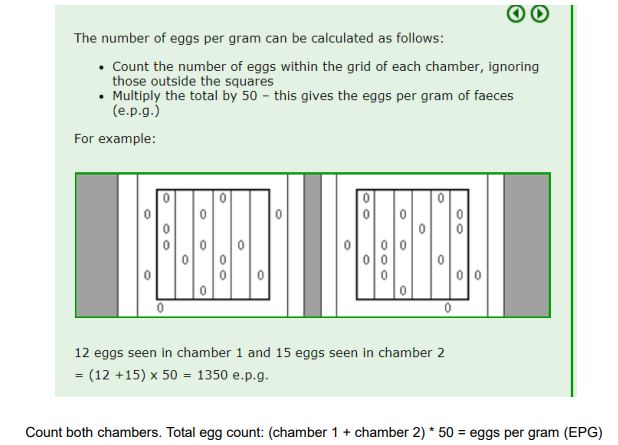
Count eggs/oocysts inside the squares (2 squares with 6 columns each)
EPG/OPG/LPG = nr of eggs in 1 and 2nd chamber x 50 if counted in both squares (100 if only one chamber)
Will most likely only see Eimeria spp. (show)
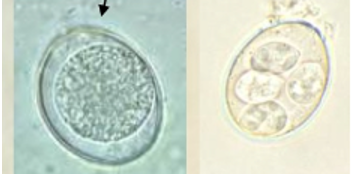
questions to most likely get:
What is the reason to why we do quantification method?
gives us indication of how many eggs per gram is in feces, to know the extent (degree) of infection, which is needed for treatment.
What is mild, moderate, severe infection?
mild - EPG (500), OPG (less than 500)
Moderate - EPG (800-1000), OPG (500-1000)
Severe - EPG (1500-2000), OPG (more than 1000)
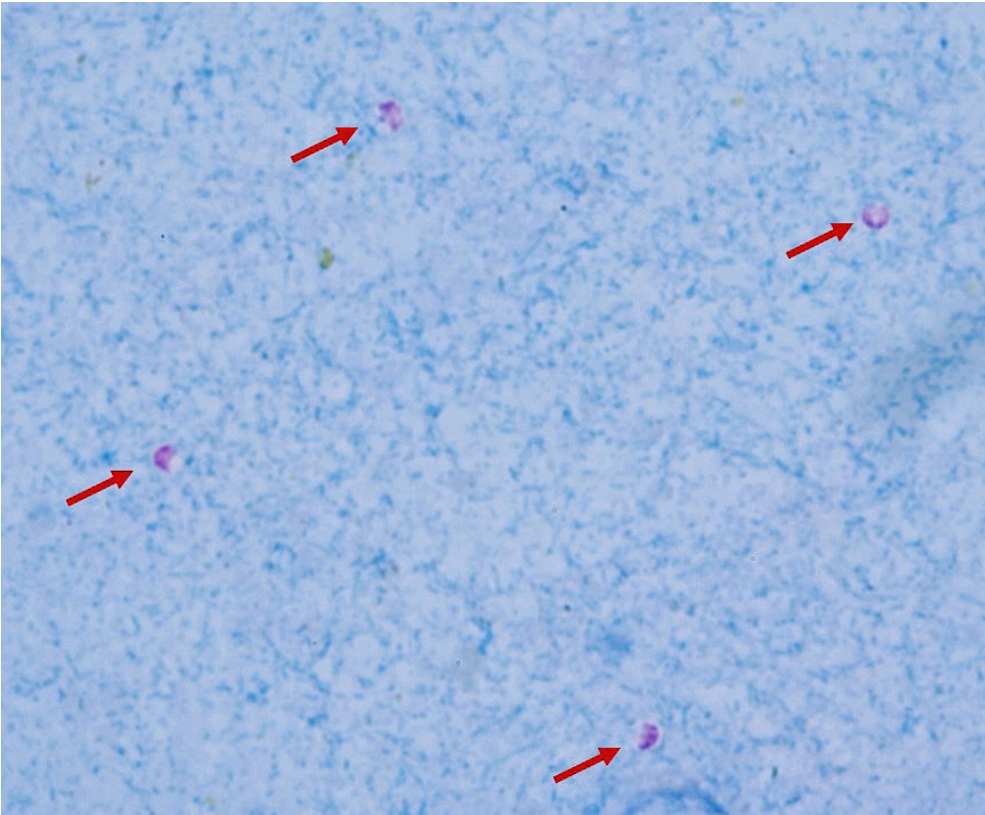
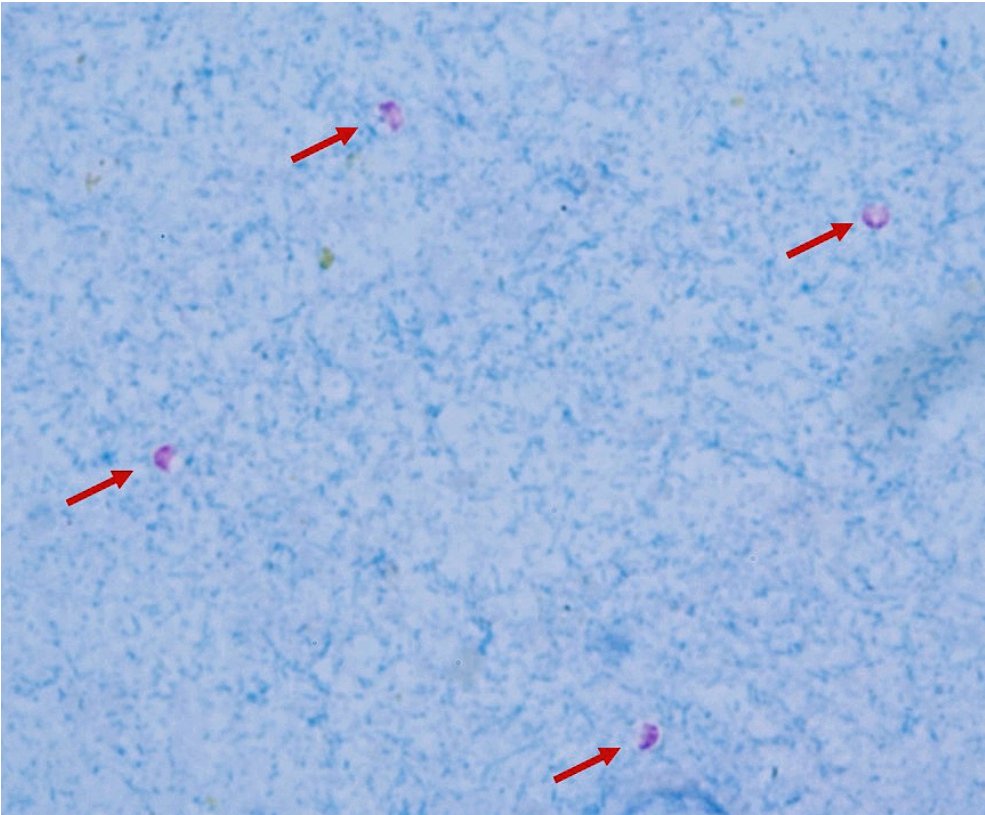
Diagnostic methods of cryptosporidiosis.
picture of what some have got earlier - what is it?
Cryptosporidium parvum has little host specificity, can infect many different species of mammals and birds but not between mammals and birds.
Main species:
Mammals: total of 18 species.
C. hominis – Humans
C. parvum – Ruminants, human, rodent
C. bovis – cattle, sheep
C. andersoni – Ruminants (located in abomasum)
C. suis – pig
C. canis & felis – car
C. muris – mice, birds
Birds: 3 species
C. baileyi – chicken (located in respiratory tract, intestine, bursa fabricii)
C. galli – chicken (located in stomach)
C. meleagridis – turkey (located in intestine, also other organs)
They have no micropyle or sporocyst, the 4 sporozoits are free within the egg. They are always embryonated as sporulation is endogenous.
Diagnosis:
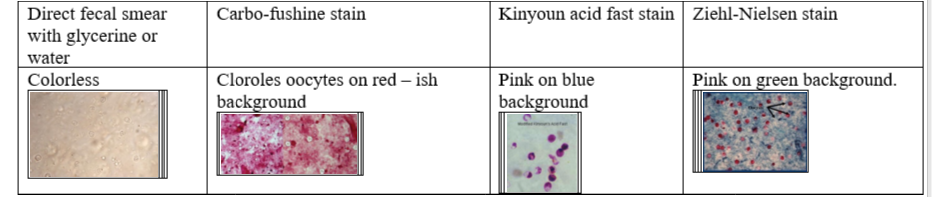
They are too small (only 5-7um) to be seen in fecal flotation method, so we need to stain them. Either by
direct fecal smear with glycerine or water - colorless
3g feces + 5m water → filer → 2-3 drops on slide
stained fecal smear with carbo-fushine stain - colorless on red-ish background
stained fecal smear with kinyoun acid fast stain - pink on blue background
stained fecal smear with Ziehl-neelsen stain - pink on greenish background
Can also be detected by antigens by rapid tests, ELISA or PCR. On staining we see them with a pink half-moon shape in the oocyst.
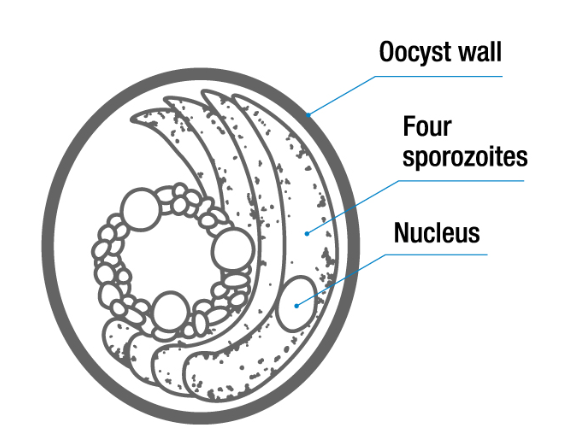
They are S, round, have 2 thin shells, embryonated and transparent color.
Tidligere: ferdig preparat med cryptosporidium egg, kin youn stain.
QS: life cycle to cryptosporidium, draw egg, 5 criteria.
egg: oocyst, S, spherical, 2 thin shells, sporulated - 4 loosely attached sporozotoites.

Diagnostic methods of trichinellosis.
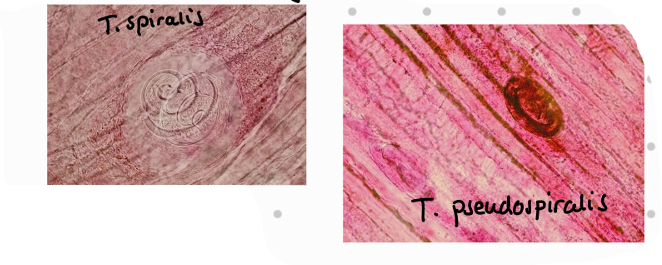
tidligere: ferdig preparat - what is in the picture?
Trichinellosis is a disease caused by the larvae of Trichinella spp, which can affect many species including humans. People can become infected by eating raw, undercooked or processed meat from pigs, wild boar, horses or game that contain trichinella.
There are 8 different species and 3 genotypes.
Trichinella spiralis – infect omnivores, carnivores, rodents, marine mammals and humans.
T. nativa – can resist -40 C, infect foxes, cats, polar bear
T. britovi – wild carnivores and omnivores
T. pseudospiralis – man and raccons, does not form capsules inside muscles.
T. murelli – USA
T. nelsoni – hyena, fox, dog
T. papuae – Papua New Guinea, does not form capsules.
T. zimbabwensis – Zimbabwe
T6 – north America subartic areas and Canada
T8 – South Africa and Namibia
T9 – Japan
Most common in Europe, North America and Asia. Females are viviparous and can produce 1500 L1 during 4-16 weeks (life span) before explusion by the host immune system.
LC: Adults develop in small intestine, females give birth to L1 → L1 migrate through lymph and blood and into the striated muscles but can be carried to all parts of body. Here they stay free or encapsulates. FH gets infected by eating an infected animal. Eg. humans eating undercooked meat from infected pig.
Diagnostic method:
Trichinoscopy is not allowed anymore - not preferred for routine meat inspection due to low sensitivity compared to digestion method (0.5g sample + a drop of 10% lactic acid compressed between two glass plates until they become translucent).
Digestion method is used for domestic swine and horses. Samples are taken from slaughter house and sent to a laboratory to be broken down and examined for the presence of Trichinella.
Predilection sites are different in species
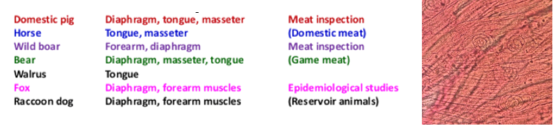
Samples should ideally be taken from the pillar of the diaphragm (good blood supply), cutting along the thick meaty parts as close to the ribs as possible. A muscle sample of at least 10g should be cut as soon as possible after death, free of fat and other tissues.
Can also be detected with ELISA in humans.
Procedure
Sample is cut into small pieces and mixed with a food processor with 40ml of digestion fluid of 40-46 degrees.
The homogenous mixture is added to a beaker with a magnet and electromagnetic stirrer for 30 minutes (imitates digestion)
filtrate through sieve into sedimentation tank/funnel and leave to settle for 30 min
Remove supernatant and add warm water 40-46C and let sit for 15-20 minutes. Repeat until the supernatant is clear
Add sediment to a petri dish or slide and examine under microscope
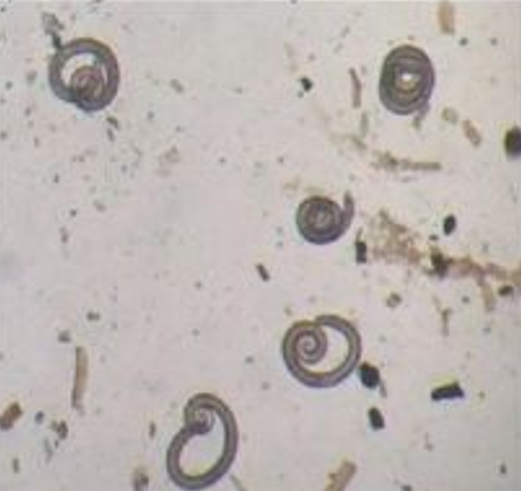
qs: Which one lives the longest in the environment? - T. nativa, T. genotype 6 (years, survive freezing).
Diagnostic methods of ruminants blood parasites.
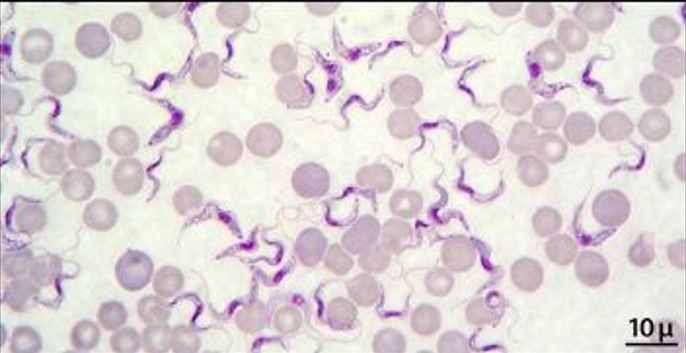
Trypanosoma – diagnosed by:
Identification of parasite in blood – trypomastigotes
Direct demonstration by wet mount or giemsa stain
ELISA, PCR
Babesia in cattle: B. bigemina, B. major, B. bovis, B. divergens
Infective stage for IM host: Sporozoits
Infective stage for FH: Erythrocytes with merozoits
Diagnosis is based on clinical signs and blood sampling.
Blood is taken from capillary blood from ear or tail tip. For B. bigemina and B. divergens take blood from vena jugularis alternatively to capillary blood. Blood is then stained by Giemsa Romanowksy
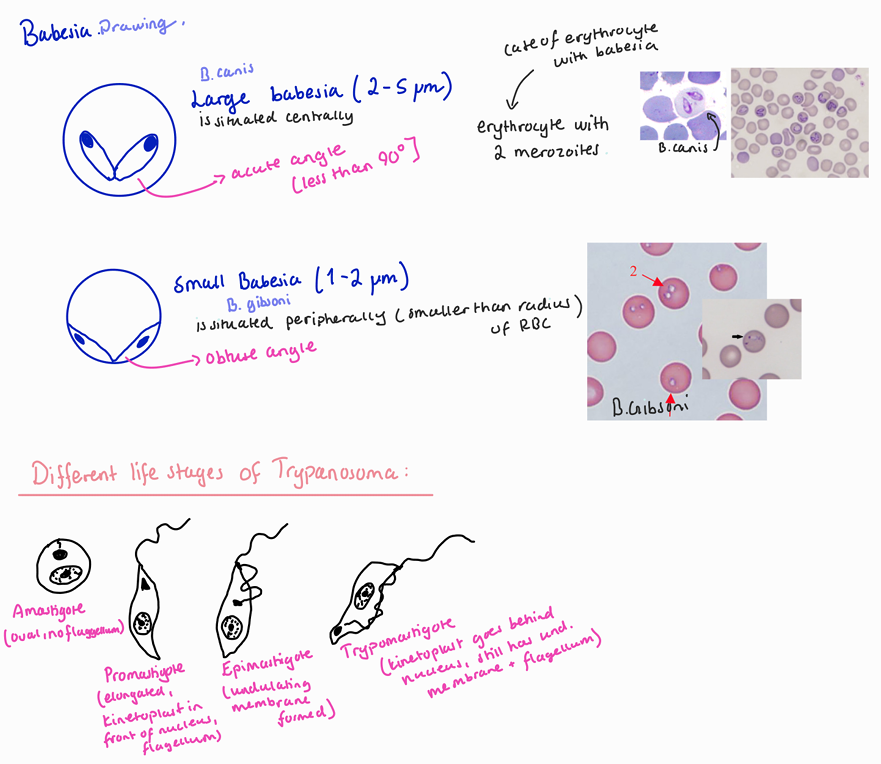
Large babesia–2-5um, center of Ec, acute angle
Small ,babesia–1-2um, peripheral of Ec,obtuse angle
Can also be diagnosed by serological method like ELISA, IFAT and PCR.
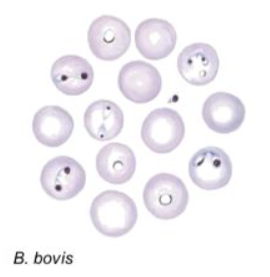
What will they ask?
Life cycle:
sexual cycle in tick host: tick eats gametocytes during blood meal on mammalian host → male + female gates in gut → zygote (gamogony) → meiosis → kinete develops.
trans-ovarial or trans-stage transmission: in female tick, kinete → ovaries + eggs → egg laying → hatch and infected with babesia → saliva. whereas by trans-stage, kinete → tissues, multiplies → salivary glands → sporogony → sporoblast → sporozoites → infect next victim.
Asexual cycle in mammalian host: sporozoites inva RBCs → trophozoites (divide by binary fission) → merozoites → new trophozoites + merozoites → some merozoites become male + female gametocytes within RBCs → gets into tick while feeding.
Species and vectors: tick is vector, mammals is host.
You will need to draw the small and large babesia and the different morphological forms of trypanosoma
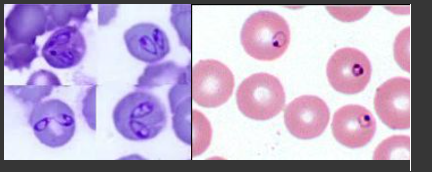
Diagnostic methods of carnivorous blood parasites.
Babesia
Stained blood smears (Giems-romanowski stain, Diff-wuick, pappenheim)
Serology
Large Babesia (2-5 um)
Babesia canis canis- Dermacentor reticulatus (Europe, Asia)
Babesia canis vogeli- Rhipicephalus sanguineus Less pathogenic!
Often without clinical signs, but fatal in puppies
Babesia canis rossi - Haemaphysalis leachi Most pathogenic! (South-Africa)
Pyriform organisms, pear-form in RBC.
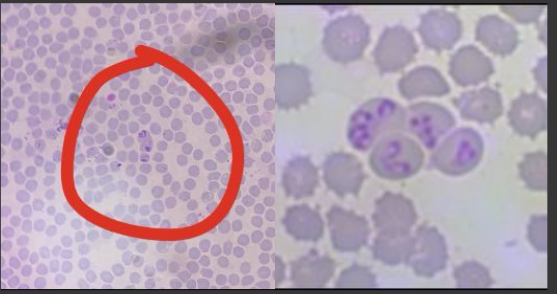
Small babesia (1-2 um)
Babesia canis gibsoni - Rhipicephalus (Europe, Africa, Asia, America)
Single form organisms
Hepatozoon (canis, felis, americanum)
Blood smear, detection of merozoites in neutrophils
Muscle biopsy
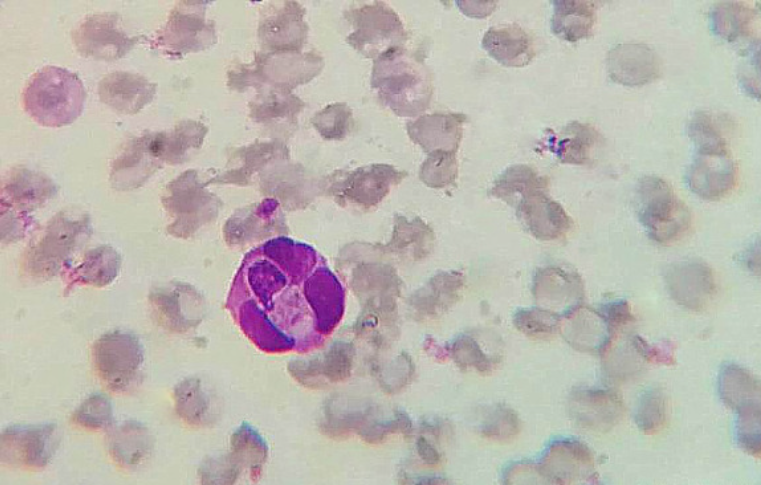
Dirofilaria immitis
Blood smear, detection of microfilariae
Knotts test
Based on clinical signs
Serology most used, ELISA and snaptest
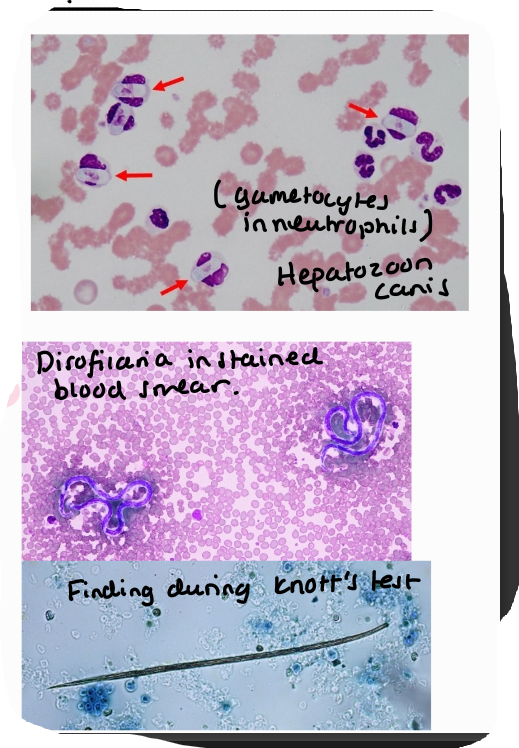
other:
plasmodium - malarie, vivax, falciparum
Leishmania - tropical, major-cutaneus, bonovoni, infantum chagasi - visceral
Trypanosoma - brucei brucei (africal sleeping sickness, salivaria), cruzi (americal chagaz disease - stercoraria)