Halogens ~ Chemical Properties
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Where is halogens found in the PT ?
In group 7 + ‘ salt former ‘
What is the oxidation number for halogen ?
Always - 1
Expect when it reacts with more reactive halogen or oxygen / peroxide
What color is Cl 2 chorine + state of matter
Cl2 is a pale green gas at room temp
What colour is Bromine Br 2 + state of matter
Dark red liquid at room temp ( only liquid non - metal )
Very volatile and readily form a red vapour
What colour is I2 iodine + state of matter
Forms a shiny black crystals at room temp
When warmed , iodine crystal sublime ( turn directly to a gas ) forming purple vapour
Compare other halogen with F - F bond
The F - F bond is very weak.
Cuz the fluorine atoms are very small and there’s a lot of repulsion between the bonding electrons
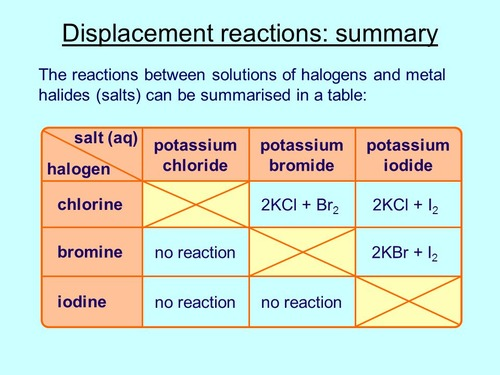
What does the term immiscible mean ?
when 2 liquids dos not mix together
What does displacement reaction mean ?
When a more reactive element displace a lower reactive element. It replaces the lower reactive element
Examples of displacement reaction in group 7
Potassium Chloride + Iodine. —→ Potassium iodide + Chlorine
Potassium Chloride + Bromine. ——> Potassium bromide + Chlorine
Potassium Bromide + Iodine. ——→ Potassium iodine + Bromine
Trends in existing ability ~ Halogen
Oxidizing ability of halogen decrease down the group , cuz atoms become larger ( less electronegative )
Halogen react by gaining electrons, which means they are oxidizing. THEMSELVEs are already reduced.
Chlorine will displace bromine
Cl2 + 2 NaBr —> 2 NaCl + Br2
Ionic equation : Cl 2 + 2 Na+ + 2 Br - ——→ Br2 + 2 Na+ + 2 Cl-
What is the Oxidising and reducing agent in the displacement of chlorine & bromine ?
Oxidising agent : Chlorine Cl2
Reducing agent : Bromide 2Br -