Chemistry to Ions Gr. 9 Science
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
Matter is anything that has ____ and ______
mass and volume
Mass is defined as __________
the amount of matter in a substance
Volume is defined as __________
the amount of space a substance or an object occupies
What is the basic unit of matter?
atom
Atoms are made of….
protons, neutrons, and electrons
Neutrons and protons are made of….
quarks
Elements contain….
only one type of atom
Elements can be found in ….
The Periodic Table
Elements can be….
single atoms or molecules
What’s an example of an element?
Oxygen
Compounds contain ….
two or more types of atom (elements)
Compounds can be…
molecules or giant structures
A chemical change is a…
change in matter that occurs when substances combine to form new substances
What is a new substance?
A substance that has different properties from those of the original substance
Most chemical changes are…
irreversible (but not all)
Physical changes are …
reversible
Water boiling is a _____ change
physical
Making toast is a ______ change
chemical
Signs of a chemical reaction:
precipation (solid formed from 2 liquids), bubbles/fizzing, colour change, temperature change, light/heat given off
What are the 4 states of matter?
solid, liquid, gas, plasma
Solids have a….
definite shape and volume
Liquids take the shape of its….
container/surroundings
Liquids have a….
definite volume
Liquids form a ____ in its container
surface
The shape and volume of gas is determined by ….
its surroundings
Plasma is an….
ionized gas
______ is an example of plasma
lightning
The ___________ and the _________ combine to form the basis of our understanding of how matter behaves
Particle Model of Matter, Kinetic Molecular Theory
The four main points of the Particle Model of Matter are:
All matter is made of very small particles
There are spaces between the particles, and the amount of space depends on the state of matter
These particles are always moving
The particles are attracted to one another. This attraction depends on the type of particle.
Solid —> Liquid
Melting
Liquid —> Solid
Solidification
Liquid —> Gas
Evaporation
Gas —> Liquid
Condensation
Gas —> Plasma
Ionization
Plasma —> Gas
Deionization
Gas —> Solid
Sublimation
Solid —> Gas
Deposition
The atom is made up of _ subatomic particles
3
The symbol for protons is _
p
The symbol for electrons is _
e-
The symbol for neutrons is _
n
Protons have a charge of _
+1
Neutrons have _____
no charge
Electrons have a charge of _
-1
+ve and -ve charges attract eachother, therefore ____________ attract eachother
protons and electrons
________ are found in the nucleus
Protons and neutrons
______ are found in energy shells
Electrons
________ is a region at the center of the atom
The nucleus
Electrons occupy…..
distinct electron shells/energy levels around the nucleus
The space that electrons take up accounts for ____ of an atom’s volume
99.99%
The mass in matter comes from _____ and ______ in the ______
protons, neutrons, nucleus
Protons have a relative atomic mass of _
1
Neutrons have a relative atomic mass of _
1
Electrons have a relative atomic mass of _
0
The volume of matter comes from ______ in the ________ that surround the nucleus
electrons, energy shells
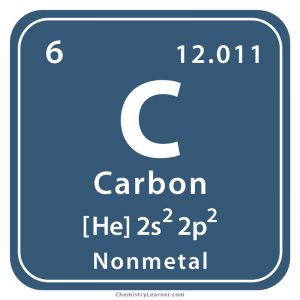
This atom’s ______ is 6
atomic number
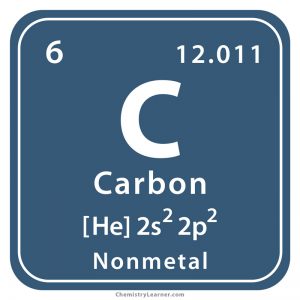
This atom’s ______ is carbon
element name
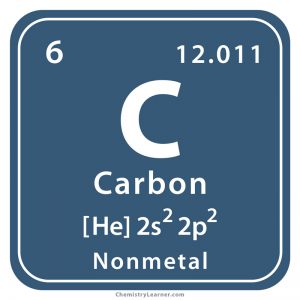
This atom’s ______ is C
symbol
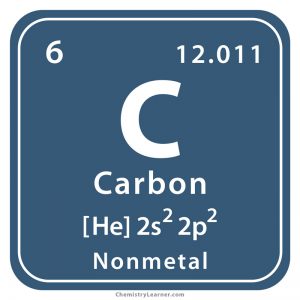
This atom’s _______ is 12.011
atomic mass
atomic _____ = number of _____ = number of ______
number, protons, electrons
number of neutrons =
atomic mass - atomic number
Isotopes are ______ of a particular chemical element which differ in neutron number.
variants
All isotopes of a given element have the same number of _____ in each atom.
protons
Electrons always fill the ____ _____ shell first, then fill the _____ shells.
inner most, outer
The inner shell has the _____ energy level.
lowest
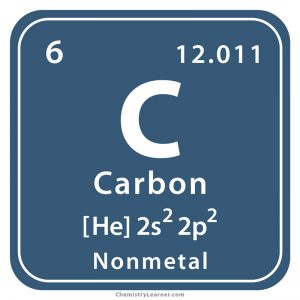
What is the electronic configuration of Carbon?
2,4
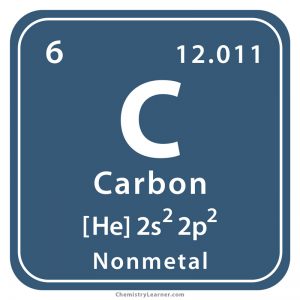
What is the electronic structure of Carbon?
6e-
An ion is…
an atom or group of atoms that has an electrical charge, either positive or negative.
_____ don’t have an overall charge.
Atoms
Why do atoms not have an overall charge?
Because they always have an equal number of electrons and protons.
How does an atom become an ion, and become charged?
When they gain or lose electrons
Why do atoms become ions?
So they can obtain full outer shells and stabilize themselves
Are ions and atoms the same thing?
No
When an atom loses an electron, it forms a _____ ion
positive
When an atom gains an electron, it forms a ______ ion
negative
Atoms with few valence electrons find it easier to ____ electrons
lose
Atoms with more valence electrons find it easier to _____ electrons
gain
Metals tend to _____ electrons and form _____ ions
lose, positive
Non-metals (except for noble gases) tend to _______ electrons and form _____ ions
gain, negative
Elements in the same group often form ions with ____ _______ charge
the same
How many valence electrons do noble gases have (group 18)?
8 (except for helium —> 2)
All the elements in the same group have the same number of ______ _____
valence electrons
All the elements in the same period have the same number of _____ _______
energy shells
The number of valence electrons go ____ by ____ as you go from group 1 to the last group of the period
up, 1
groups = ____
columns
periods = ______
rows
The number of shells ____ by ____ as you move down the first _______
increase, 1, period
What does the bolded line that zigzags through the periodic table for?
To separate the metals from the non-metals in a clear way
Atomic number _______ as you go from ____ to _____
increases, left, right