Polar/NonPolar Quiz
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
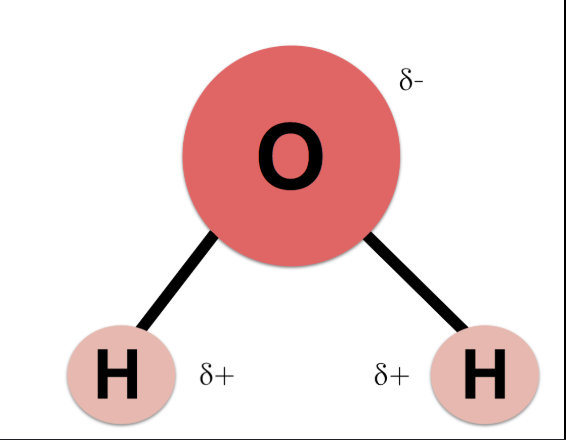
H2O
polar because of hydroxyl group, bent shape, different EN
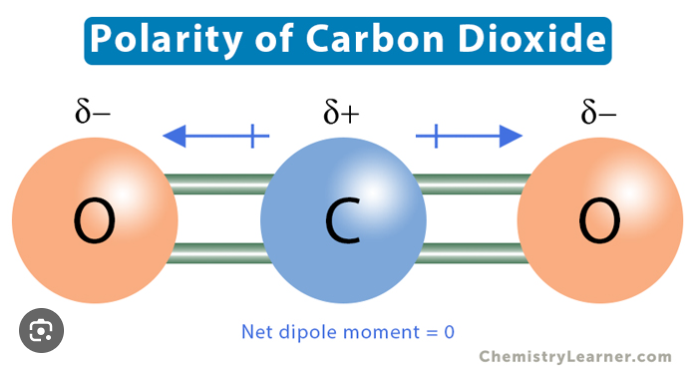
CO2
nonpolar because although indiv. O-C bonds r polar, there is a symmetrical shape
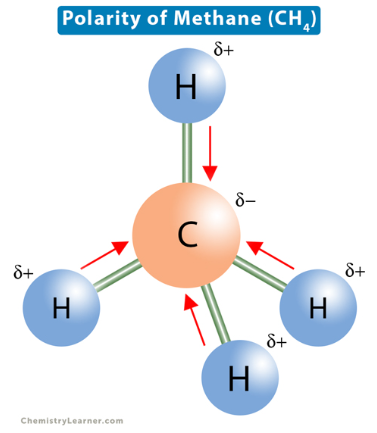
CH4 (methane)
nonpolar because C-H bonds have similar EN; bonus explanation: C is .37 more EN than H, so C pulls H towards it, giving C a + charge and H a - charge. The difference in charge makes the molecule polar. BUT symmetry CANCELS ALL DIPOLE MOVEMENT (also w/ carbon tetrachloride, CO2)
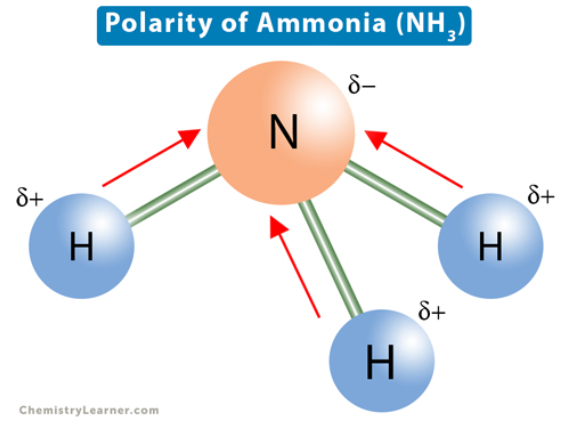
NH3 (ammonia)
polar because N-H has significantly different EN
C2H5OH (ethanol)
polar because has OH group
O2
nonpolar bc similar EN
CH₃COCH₃ (acetone)
polar bc has a carbonyl group (O-C)
C₆H₆ (Benzene)
nonpolar bc C-C has similar EN, and whole thing is C-H bonds & symmetrical
HCl (Hydrogen Chloride)
polar bc different EN
CH₃Cl (chloromethane)
polar bc difference in EN (C-Cl)
asymmetric
Is Polar asymmetric or symmetric?
symmetric
Is Nonpolar asymmetric or symmetric
O-C and O-H
what bonds are polar?
O has more EN than C
why is O-C polar?
C-H and C-C
what bonds are nonpolar?
when 2 elements have similar EN
when is something nonpolar?
Phosphate (PO4^ 2-), carboxyl (COOH), amino (NH2), hydroxyl (OH)
What functional groups are polar?
Polar due to the significant difference in EN of O and H atoms
If any molecule/nonpolar molecule has an OH group, what is its polarity and why?
N-H
what is a bond that has significantly different EN's
Alkyl (CH3) and aromatic rings (C6H6)
what functional groups are nonpolar?