Chemistry II Lesson 5: Chromatography
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Why use an oil bath as compared to a water bath when heating up the distilling flask during a distillation?
An oil bath is preferable because it will not evaporate and will also allow us to maintain a steady temperature throughout the distillation.
What is the purpose of the condenser in a distillation?
The condenser will cause the compound with the lowest boiling point (lower than the temperature the mixture has been heated to) to condense and collect as liquid in the receiving flask.

The purpose of a vacuum in a distillation is that it:
(A) allows you to separate substances with extremely close boiling points more accurately.
(B) allows you to separate substances with extremely distant boiling points more accurately.
(C) allows you to separate substances with extremely high boiling points without damaging the substances.
(D) allows you to separate substances with extremely low boiling points without damaging the substances.
(C) allows you to separate substances with extremely high boiling points without damaging the substances.
The purpose of a vacuum in a distillation is that it allows you to separate substances with extremely high boiling points without damaging the substances.
Why does a vacuum lower the boiling point of a substance?
A vacuum decreases the atmospheric pressure which pushes gas particles down into the liquid phase. With zero atmospheric pressure, these gas particles are more easily able to escape the liquid phase.
The purpose of doing a fractional distillation as opposed to a regular distillation is that a fractional distillation:
(A) allows you to separate substances with extremely close boiling points more accurately.
(B) allows you to separate substances with extremely distant boiling points more accurately.
(C) allows you to separate substances with extremely high boiling points without damaging the substances.
(D) allows you to separate substances with extremely low boiling points without damaging the substances.
(A) allows you to separate substances with extremely close boiling points more accurately.
The purpose of doing a fractional distillation as opposed to a regular distillation is that a fractional distillation allows you to separate substances with extremely close boiling points more accurately.
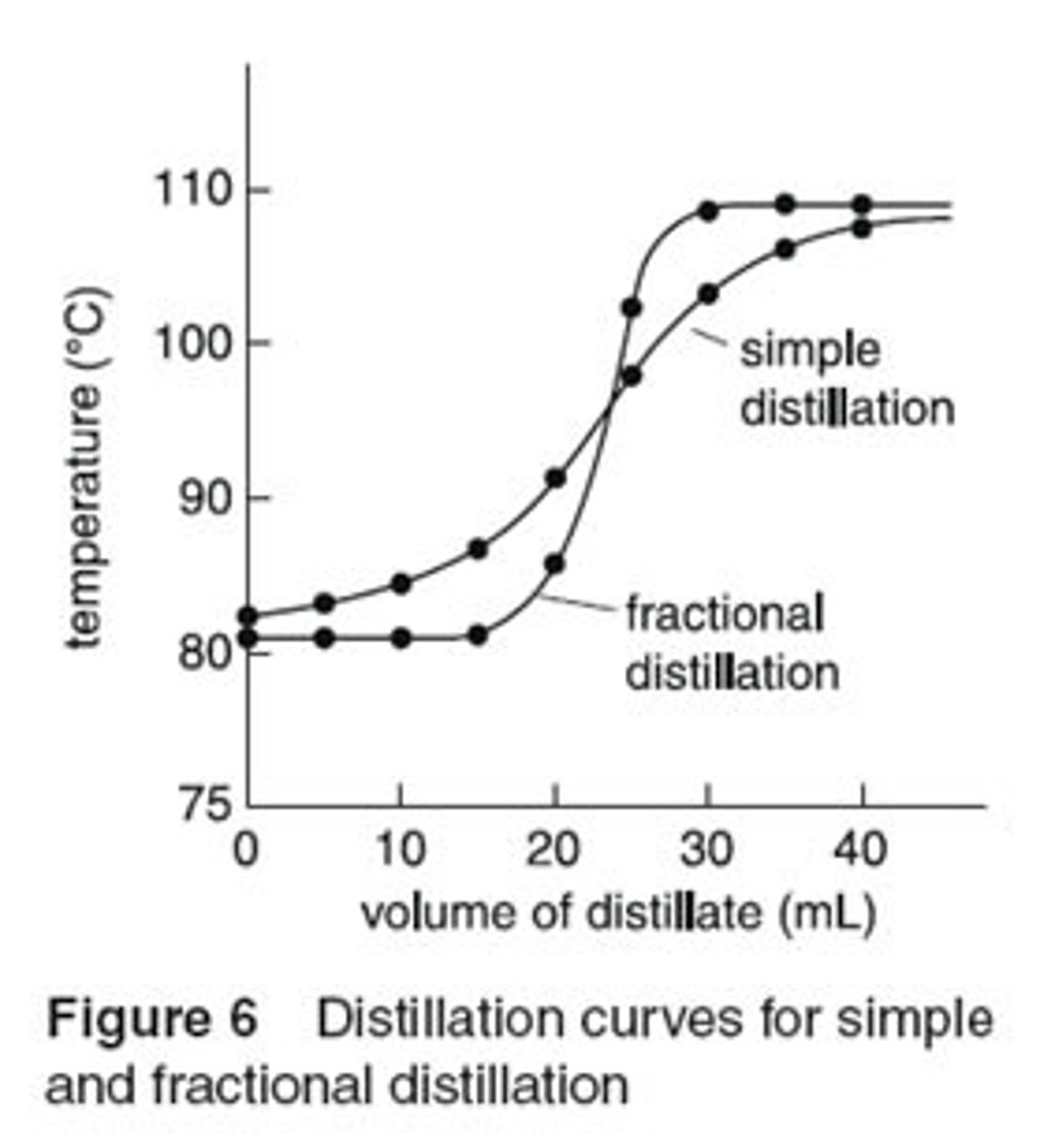
How does a fractionating column help you more accurately separate compounds with very close boiling points?
The fractionating column contains steel wool, beads, or another substance to which the gases can condense on. This way, the gases will have to condense and evaporate multiple times further away from the heat source in order to escape the distilling flask and make it to the condenser. This way, only the compound with the lower boiling point will be able to escape.
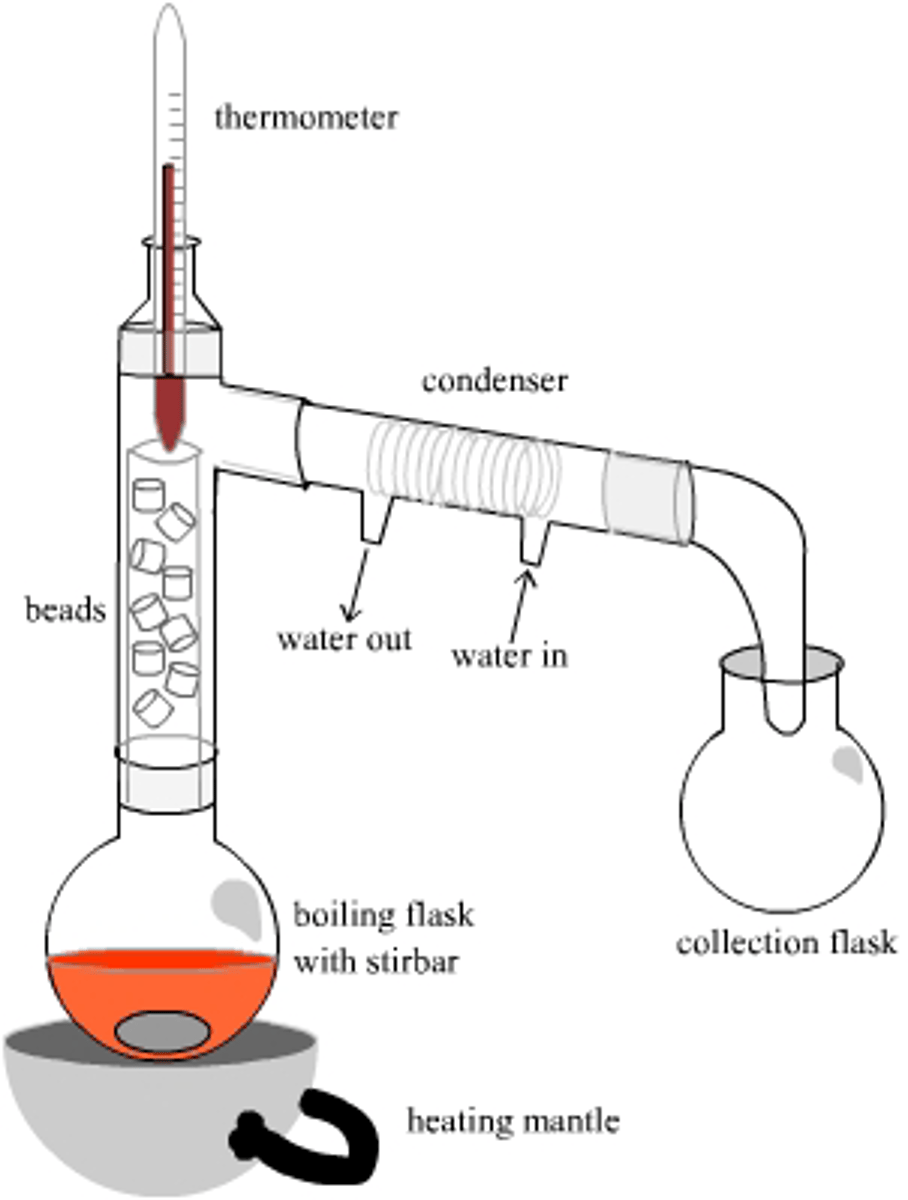
CRB How could superheating adversely affect distillation?
(A) It decreases the vapor pressure of the mixture being distilled.
(B) It could allow multiple liquids to reach boiling point, leading to an impure distillate.
(C) It can prevent a distillate from forming altogether, or lead to breaking glassware.
(D) It decreases the total yield of the distillate.
(B) It could allow multiple liquids to reach boiling point, leading to an impure distillate.
Superheating often occurs when gas bubbles cannot overcome atmospheric pressure and surface tension. That necessary increase in temperature then could cause multiple liquids to boil at the same time.
What is the purpose of an extraction?
To separate compounds based on their polarity.
Draw a simple diagram of an apparatus that could be used to do an extraction. Include the following terms:
- Separatory Funnel
- Stopcock
- Receiving Flask

CRB When choosing solvents to use in an extraction, which of the following is NOT a desired characteristic?
(A) The solvents are miscible
(B) The solvents have very different densities
(C) The solvents are volatile
(D) The solvents do not react with each other
(A) The solvents are miscible
When choosing solvents for an extraction, the solvents must be IMMISCIBLE! Without this, the two solvents could not easily be separated.
CRB After not paying attention when doing the pre-lab exercises, two miscible solvents were mixed! Which of the following could be a viable way to separate them?
(A) Extraction
(B) Affinity Chromatography
(C) Distillation
(D) Recrystallization
(C) Distillation
As long as the two solvents have different boiling points, distillation could be used to remove the more volatile solvent.
CRB You add ether and water to the Separatory Funnel. Which would be considered the aqueous and which would be considered the organic layer? Which would end up on top and which would end up on bottom?
Ether is non-polar and has a lower density than water; thus, it would be the organic layer and end up on top. Water is polar and has a higher density than ether; therefore, it would end up on the bottom.
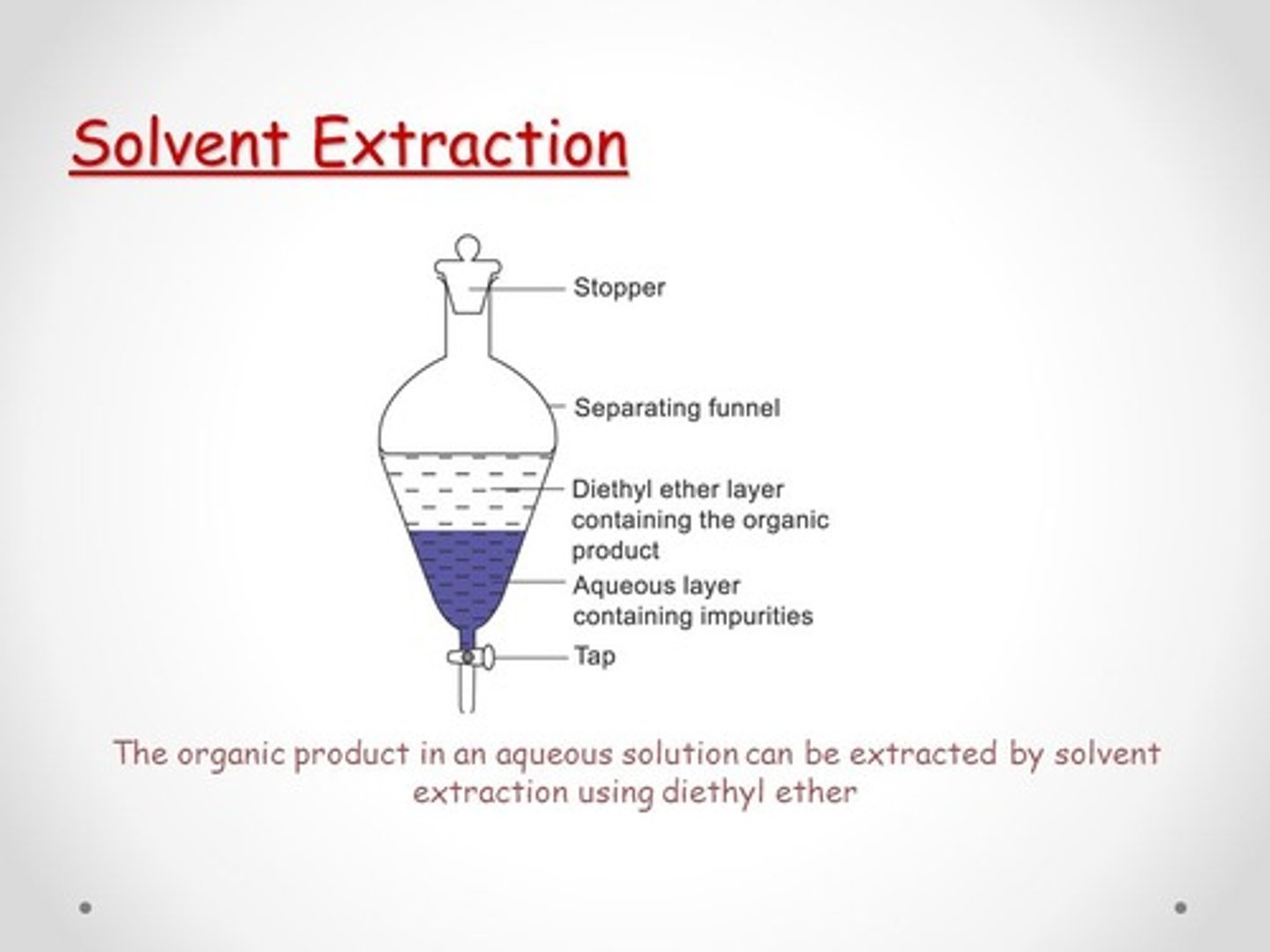
CRB If you were trying to minimize the amount of extractions needed to isolate one compound in a solvent, would you want that solvent to be on the top or bottom? Why?
To minimize the amount of extractions and pouring back into the separatory funnel, the compound to isolate should stay in the top layer. That way, you can just add the second solvent, shake, pour off that lower solvent, and repeat.
CRB If you wanted the organic layer to be on the bottom, which of the following organic solvents would you want to use?
(A) Ethyl Alcohol
(B) Ethyl Ether
(C) CH2Cl2
(D) Ethane
(C) CH2Cl2
A halogenated organic solvent will be more dense than water, and will be the bottom layer in an extraction.
You add a solution of carboxylic acid mixed with amide to your separatory funnel. Water and CH2Cl2 were already in the separatory funnel as well. You open the Stopcock until the entire bottom layer is in the receiving flask. At this point, you evaporate the solvent in the receiving flask, leaving you with your desired compound. What was your desired compound?
(A) Water
(B) Amide
(C) Carboxylic Acid
(D) CH2Cl2
(B) Amide
The Amide was your desired compound, as it is less polar than a carboxylic acid and ends up in the lower organic phase.
You evaporated the CH2Cl2, leaving your amide in the flask. The carboxylic acid is still in the separatory funnel, dissolved in the water.
CRB Now imagine the previous example wanted to isolate the amide. Which of the following would be the best procedure to fully remove the carboxylic acid?
(A) Perform one extraction than evaporate the ether.
(B) Perform one extraction, wash with ether again and evaporate the water.
(C) Perform one extraction, wash with water again, extract again, and evaporate the ether.
(D) Perform one extraction, wash with ether again, extract again and evaporate the ether.
(C) Perform one extraction, wash with water again, extract again, and evaporate the ether.
Washing with water again will remove any leftover carboxylic acid! This ensures the isolated amide will be pure.
You add ether and water to the Separatory Funnel. You then add a mixture of alcohol, carboxylic acid, and an alkane to the Separatory funnel. Describe how you might isolate the carboxylic acid from the other two compounds.
Add a base to deprotonate the carboxylic acid. This way, your carboxylic acid is far more polar than the other two substances. Once you've opened the stopcock and your carboxylic acid is in the receiving flask, evaporate the water and there you have it!
What is the purpose of chromatography?
Separate substances based on their various properties such as size or polarity.
In Paper Chromatography, the paper acts as the ____________ Phase and the solvent in the beaker acts as the _____________ Phase.
(A) Mobile, Stationary
(B) Kinetic, Stagnant
(C) Stationary, Mobile
(D) Stagnant, Kinetic
(C) Stationary, Mobile
In Paper Chromatography, the paper acts as the Stationary Phase and the solvent in the beaker acts as the Mobile Phase.
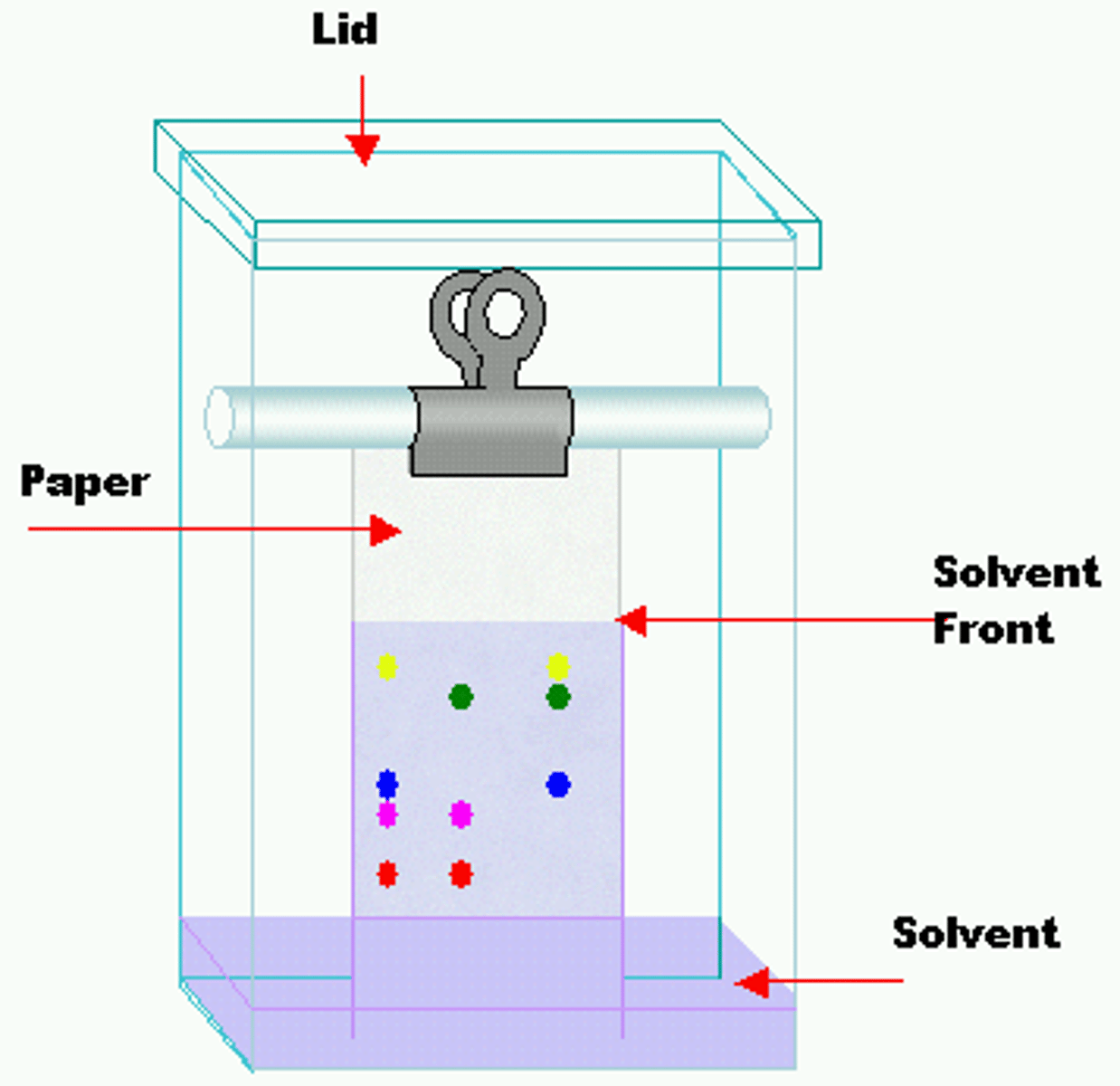
A spot will move farther in Paper Chromatography if:
(A) It is lighter.
(B) It is heavier.
(C) It is more attracted to the Mobile Phase.
(D) It is more attracted to the Stationary Phase.
(C) It is more attracted to the Mobile Phase.
This is the basis of Paper Chromatography. Our goal is to separate compounds based on their attraction to the mobile vs. the stationary phase.
What is the difference between paper chromatography and Thin Layer Chromatography (TLC)?
They are the same thing, except that in TLC, the stationary phase is always Silica Gel.
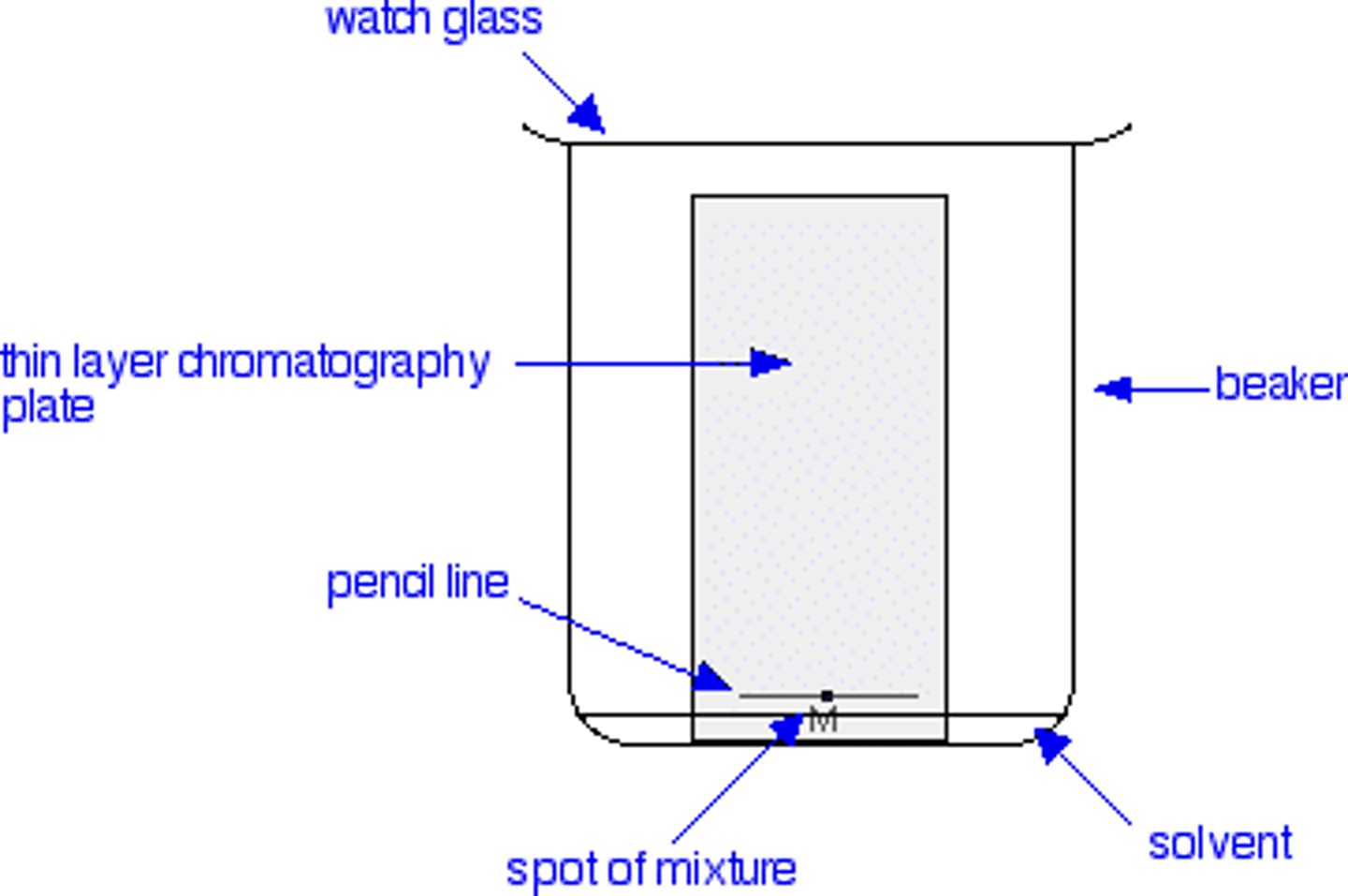
Is Silica Gel polar or non-polar?
Silica Gel is polar.
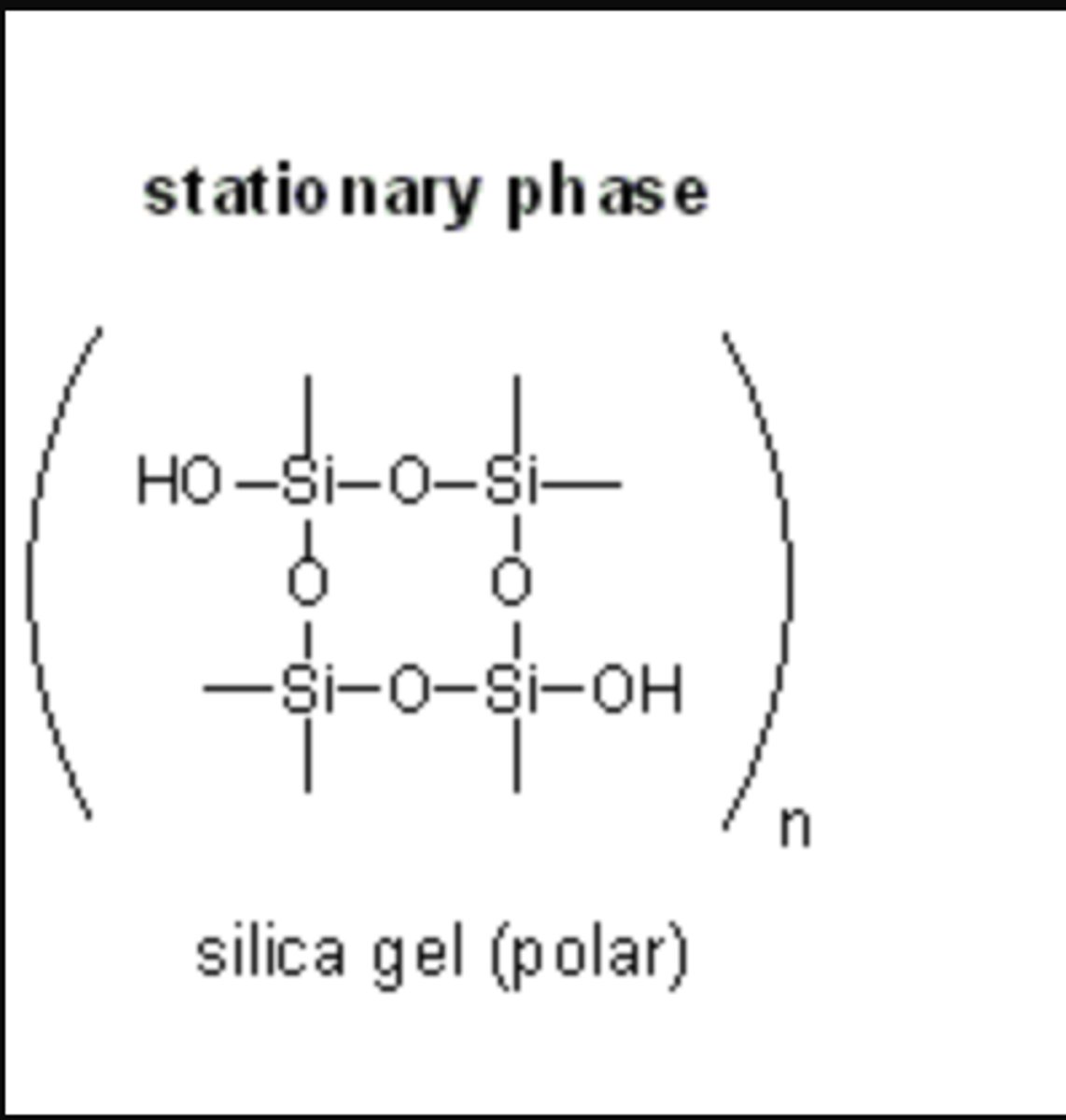
CRB In Normal Phase Chromatography, what is the typical Stationary Phase that is used?
(A) Wool beads
(B) Organic solvent
(C) Silica gel
(D) Paper
They typical Stationary Phase in Normal Phase Chromatography is Silica Gel, which is polar.
Normal Phase Chromatography features a polar stationary phase and nonpolar mobile phase.
CRB You are using Normal Phase Chromatography to separate an alcohol, an alkane, and a carboxylic acid anhydride. Line these up from eluting first (left) to eluting last (right):
I. Alcohol
II. Alkane
III. Carboxylic Acid Anhydride
(A) I < II < III
(B) II < I < III
(C) III < II < I
(D) III < I < II
(B) II < I < III
Using Normal Phase Chromatography, the most non-polar substances will elute first; thus, the elution order is as follows: Alkane < Alcohol < Carboxylic Acid Anhydride.
You are using Size-Exchange Chromatography to separate three substances. Line these up from eluting first (left) to eluting last (right):
I. Nonane
II. Butane
III. Hexane
(A) I < II < III
(B) II < I < III
(C) I < III < II
(D) III < I < II
(C) I < III < II
Size-Exchange Chromatography to separates substances based on their molecular weight, and the smallest substances will elute last as is the case here: Nonane (C9H20)< Hexane (C6H14)< Butane (C4H10)
The Khan Academy video mistakenly describes this, so see the next card for an explanation.
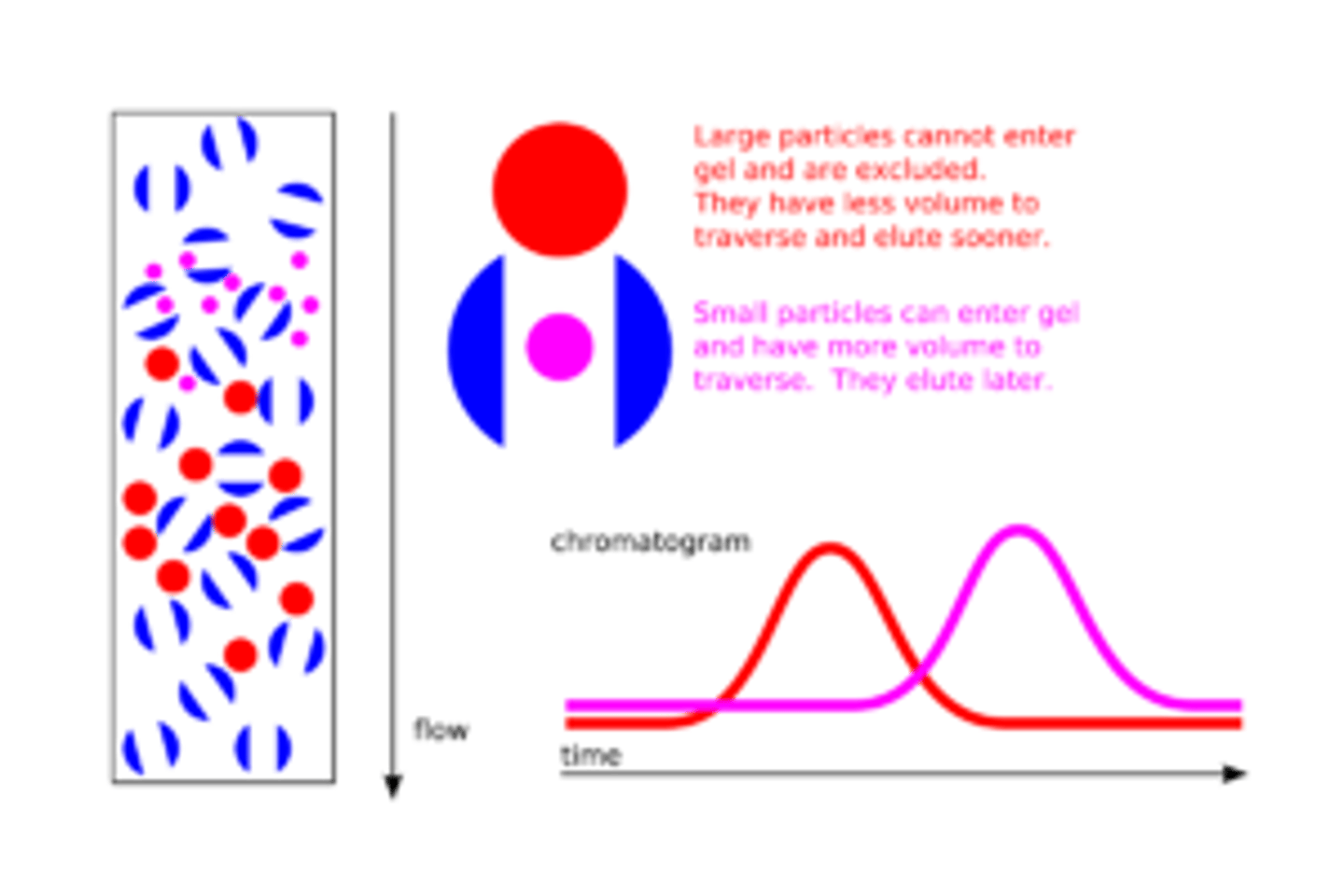
Why is it that the smaller compounds elute last in Size-Exchange Chromatography?
The Stationary Phase used in Size-Exchange Chromatography consists of beads with small holes that run through them. The smaller compounds will have to make their way through these small holes while the big compounds will go around the beads, quickly making their way through the column.
NOTE: She doesn't explain this correctly in the video.
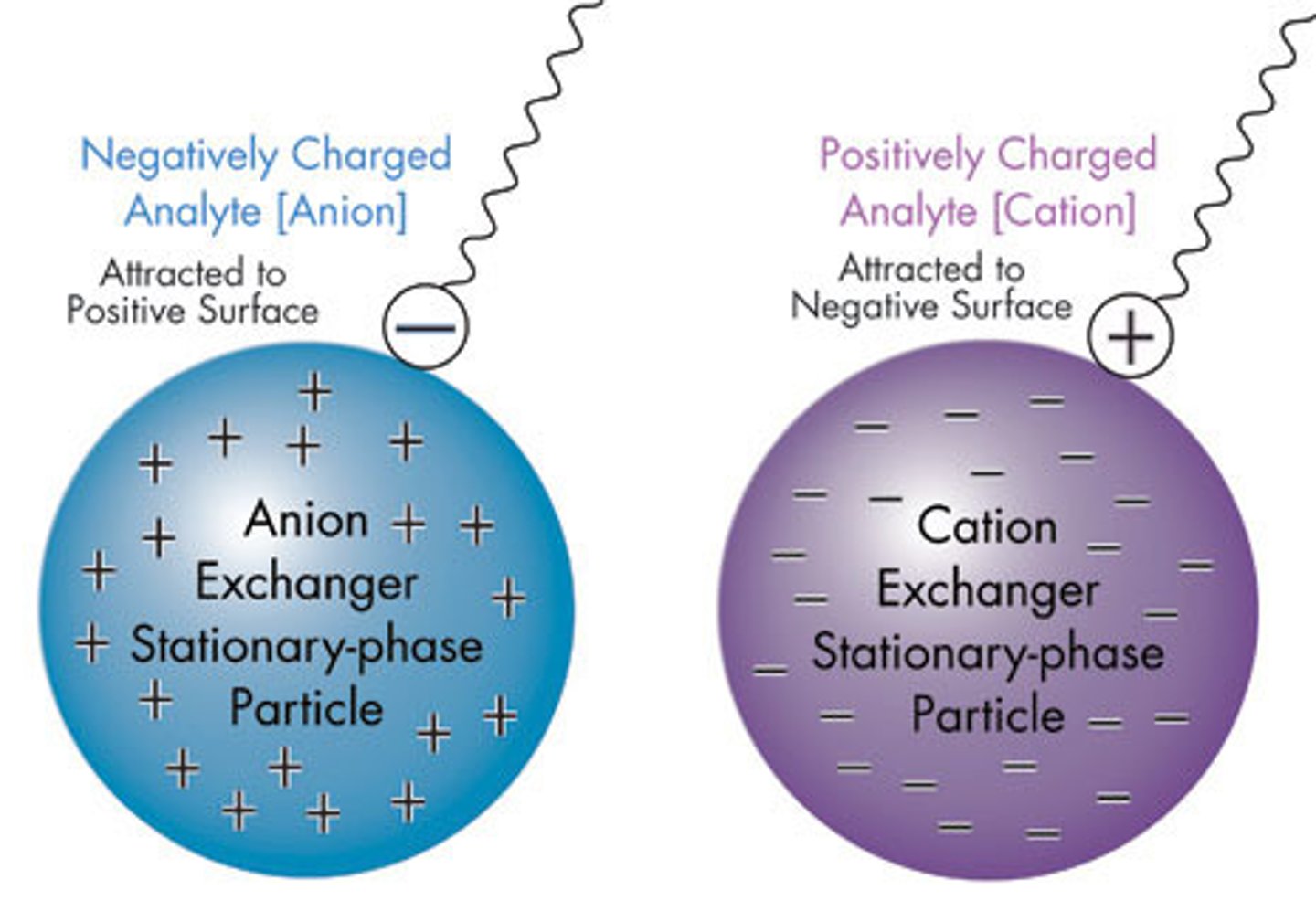
Is the Stationary Phase negatively-charged or positively-charged in Cation-Exchanged Chromatography? What about in Anion-Exchange Chromatography?
In CATION-Exchange Chromatography, the Stationary Phase is negatively charged; Thus, CATIONS will be retained in the column.
In ANION-Exchange Chromatography, the Stationary Phase is positively charged. Thus, ANIONS will be retained in the column.
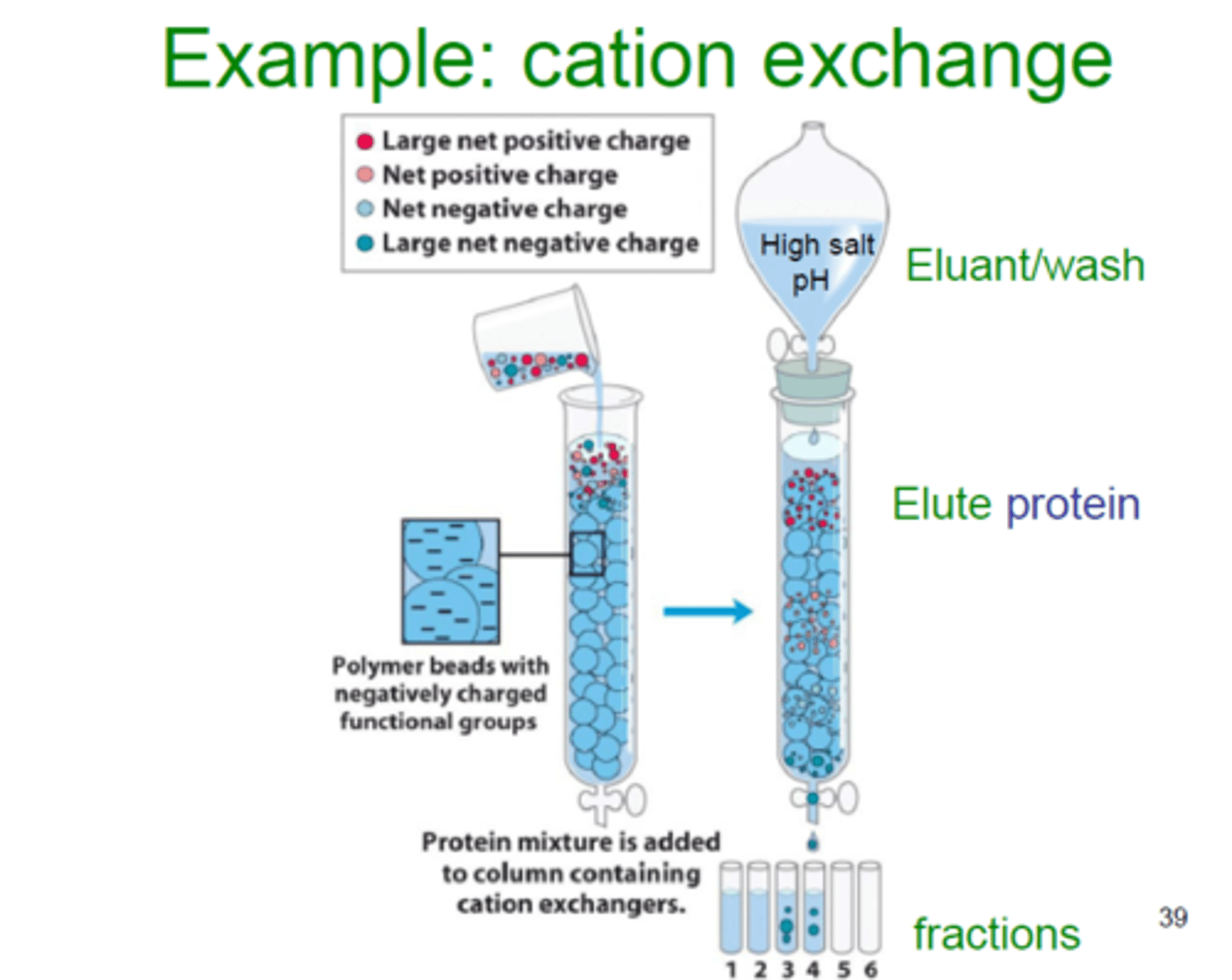
You are using Cation-Exchange Chromatography to separate three substances. Line these up from eluting first (left) to eluting last (right):
I. Carboxylate
II. Ammonium
III. 3-Hexanone
(A) III < II < I
(B) III < I < II
(C) I < II < III
(D) I < III < II
(D) I < III < II
In Cation-Exchange Chromatography, the Stationary Phase is negatively charged; thus, cations will be retained and anions will move through quickly as follows: Carboxylate < 3-Hexanone
< Ammonium
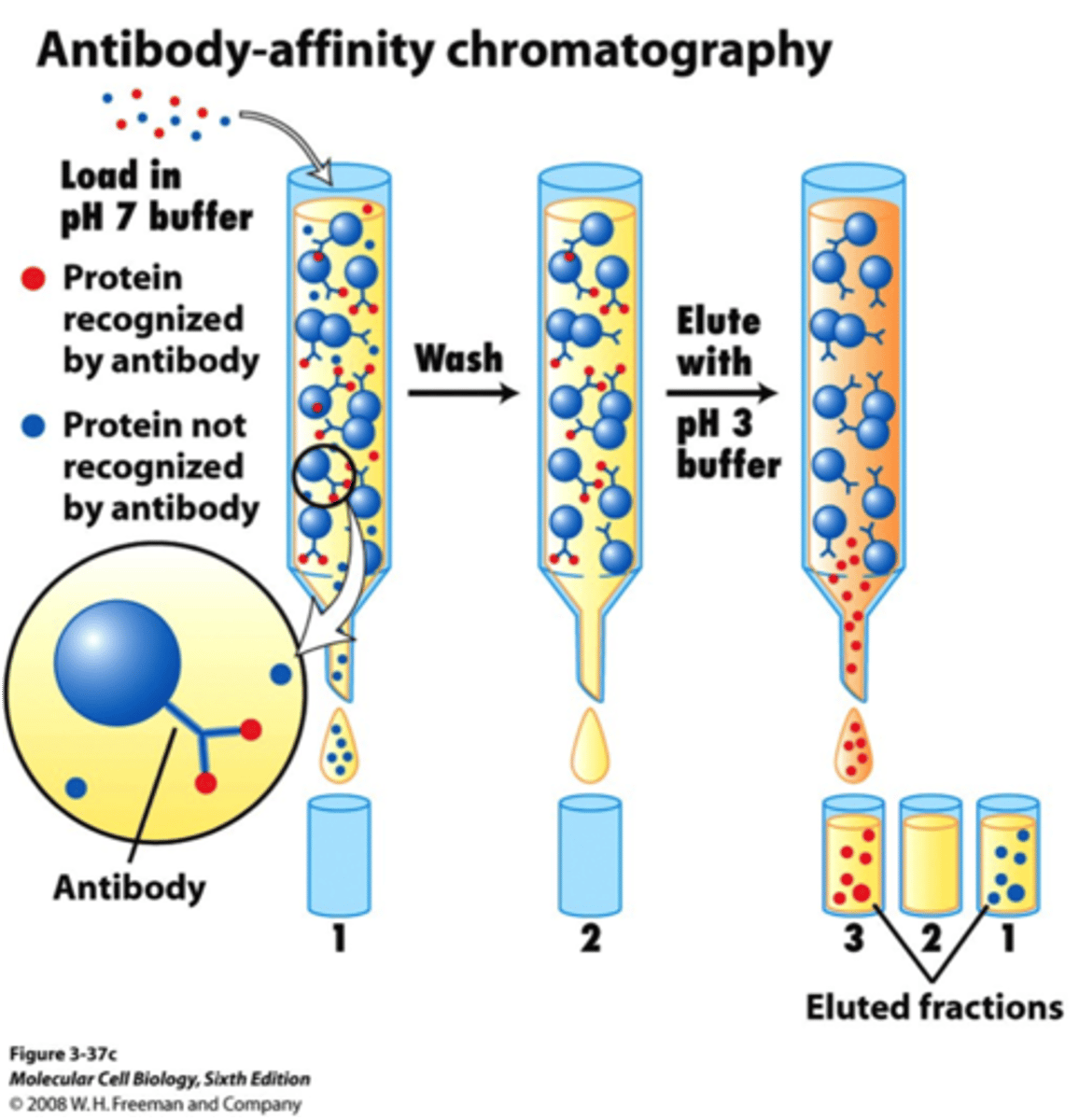
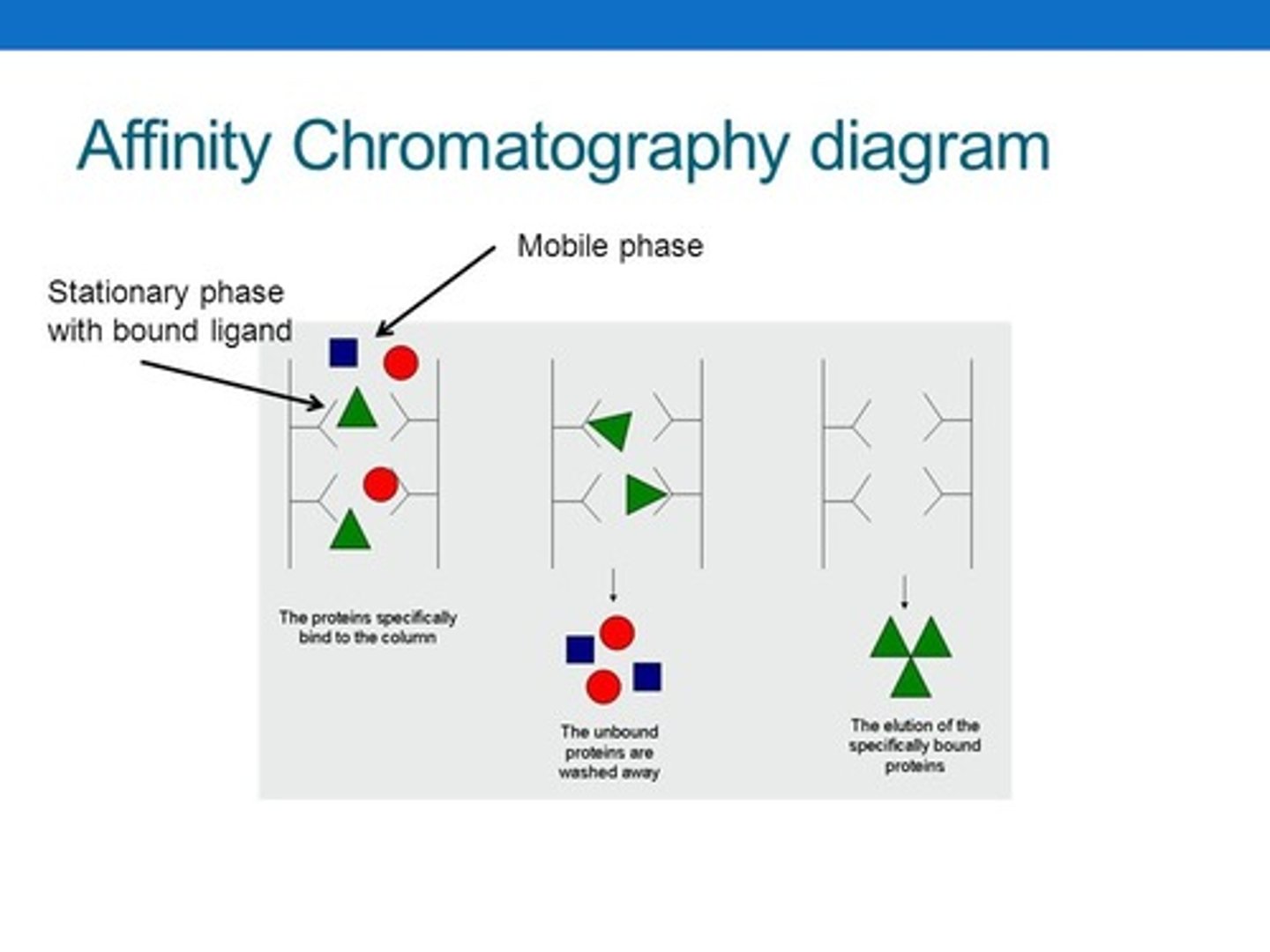
In Affinity Chromatography, what would a typical Stationary Phase be composed of? Mobile Phase?
In Affinity Chromatography, a typical Stationary Phase would be composed of an enzyme. The Mobile Phase would something the substrate is even more attracted to in order to wash it out in the end.
What makes High-performance Liquid Chromatography (HPLC) special compared to Normal Phase Chromatography?
HPLC works with very small quantities and has a much higher level of precision.
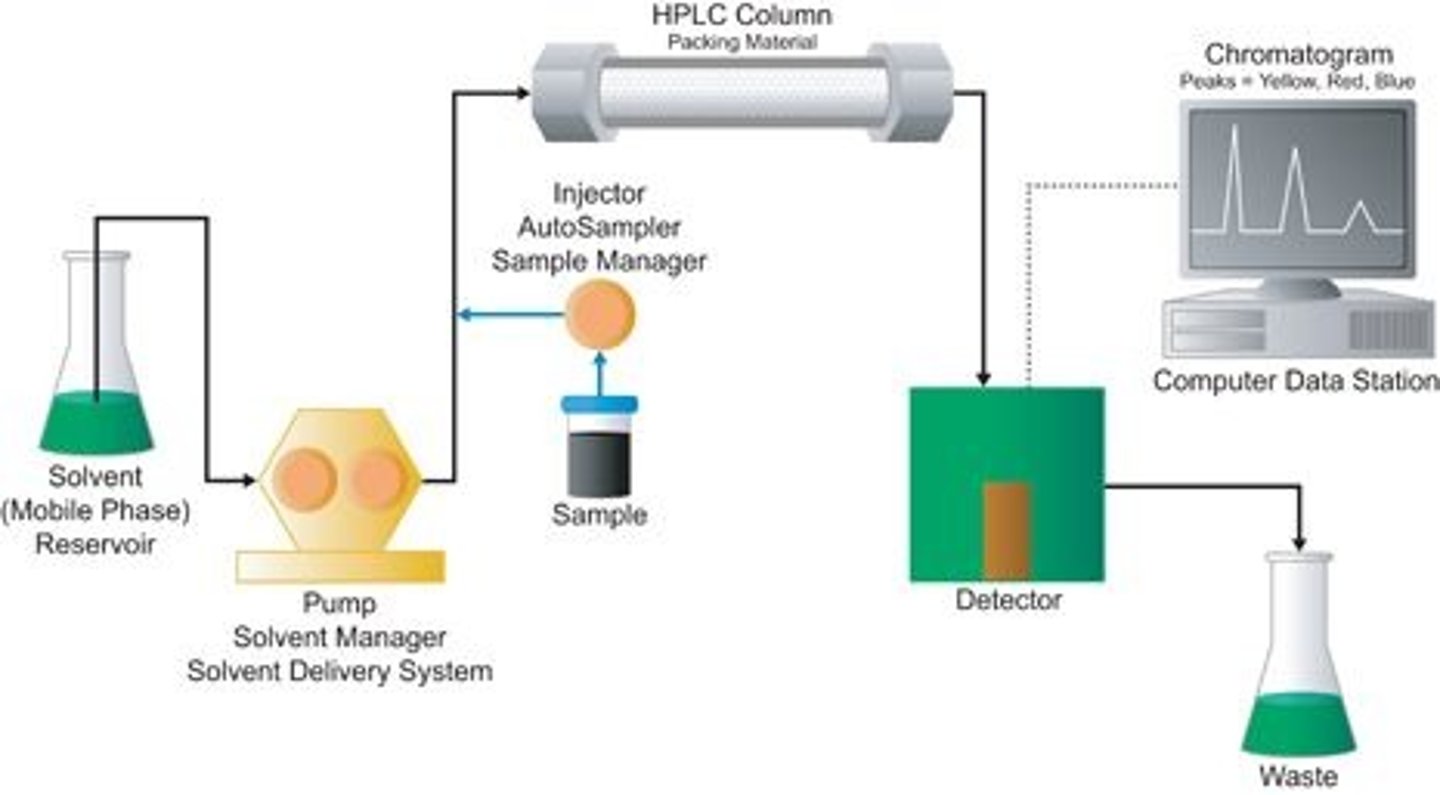
CRB Fill in the blanks: In TLC and HPLC, the more polar phase is the __________ phase. In Reverse Phase Chromatography, the more polar phase is the __________ phase.
(A) Mobile, Mobile
(B) Mobile, Stationary
(C) Stationary, Mobile
(D) Stationary, Stationary
(C) Stationary, Mobile
In TLC and HPLC, the more polar phase is the Stationary phase. In Reverse Phase Chromatography, the more polar phase is the Moblile phase.
What type of compounds will typically fluoresce using a UV lamp?
Compounds that are aromatic.
You are using Thin Layer Chromatography to separate three compounds. Line these up from least far (left) to the farthest (right):
I. Alcohol
II. Alkane
III. Carboxylic Acid Anhydride
(A) III < II < I
(B) III < I < II
(C) I < II < III
(D) I < III < II
(B) III < I < II
In Thin Layer Chromatography, the stationary phase is polar Silica Gel, which will hold on more tightly to polar compounds; thus non-polar compounds will travel the farthest as is the case here: Carboxylic Acid Anhydride < Alcohol < Alkane
What equation allows you to calculate the Retention Factor (Rf) for a given compound during a TLC?
Rf = d (solute) / d (solvent)
Rf = Retention Factor
d (solute) = Distance Traveled by Solute
d (solvent) = Distance Traveled by Solvent
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
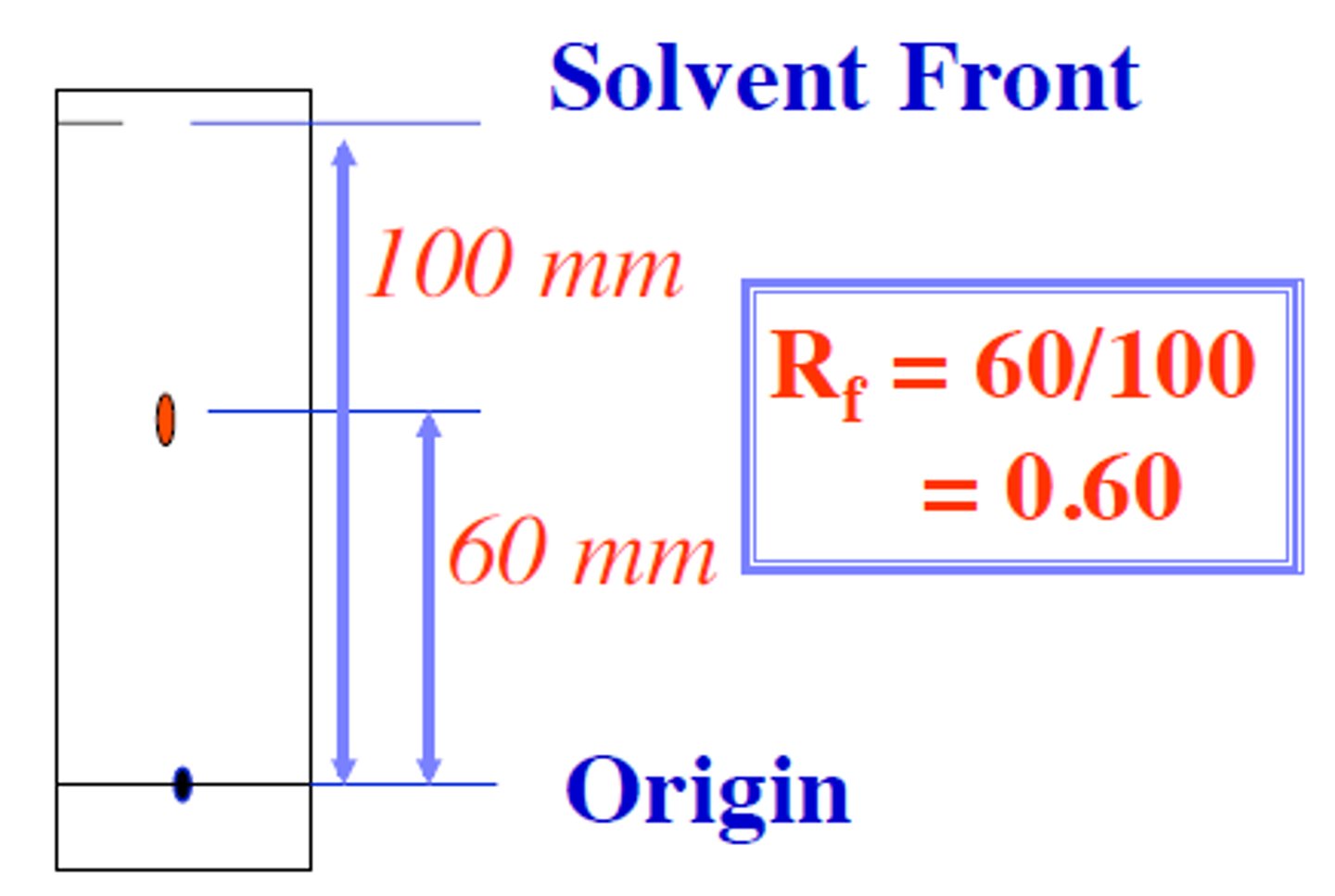
The Mobile Phase travels 8.5 units. compound 1 travels 3.2 units and compound 2 travels 6.4 units. What is the Retention Factor for Compound 1?
(A) .22
(B) .38
(C) .50
(D) 2.0
(B) .38
Rf = d (solute) / d (solvent)
Rf = 3.2 / 8.5
Rf = approx. .4 (actual: .38)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
Why is it important to keep the sand/beads of a chromatography column wet at all times?
If it runs dry, it may cause mixing of bands, which would result in a lack of separation between your compounds of interest.
What would happen if your bands ended up slanted while doing a Column Chromatography?
The bands would mix and the compounds would not separate appropriately.
In Gas Chromatography, the Stationary Phase is a __________ while the Mobile Phase is a _____________.
(A) Gas, Liquid
(B) Liquid, Gas
(C) Gas, Solid
(D) Solid, Gas
(B) Liquid, Gas
In Gas Chromatography, the Stationary Phase is a Liquid while the Mobile Phase is a Gas.
You are using Gas Chromatography to separate three substances. Line these up from eluting first (left) to eluting last (right):
I. Hexanoic Acid
II. Hexanone
III. Hexane
(A) III < II < I
(B) III < I < II
(C) I < II < III
(D) I < III < II
(A) III < II < I
In Gas Chromatography, substances are separated based on boiling point, with lower boiling point substances eluting first as is the case here: Hexane (bp = 68° C) < Hexanone (bp = 128° C) < Hexanoic Acid (bp = 401° C)
NOTE: You don't need to be told the exact boiling points. Simply keep in mind that more polar substances tend to have higher boiling points because of more intermolecular interactions.
Why do compounds with lower boiling points travel further in a Gas Chromatography column?
Compounds with low boiling points will vaporize more readily and thus get dragged along by the gas Mobile Phase while compounds with higher boiling points will not vaporize as easily and will thus remain dissolved in the liquid Stationary Phase.
Which will result in greater separation between bands when running Gas Chromatography?
I. Longer Coil
II. Higher Temperature
III. Higher Pressure
(A) I Only
(B) I and III Only
(C) II and III Only
(D) I, II, and III
(A) I Only
Longer coil, LOWER temperature, and LOWER pressure will result in greater separation of bands when running Gas Chromatography.
You run a Gas Chromatography Column on a solution of three compounds Hexanol, Hexane, and Hexanoic Acid. What is represented by the first peak in this Gas Chromatography?
(A) Hexanol
(B) Hexane
(C) Hexanoic Acid
(D) Air
(D) Air
The first small peak in Gas Chromatography typically corresponds to the air that you is let into the machine with your compounds.
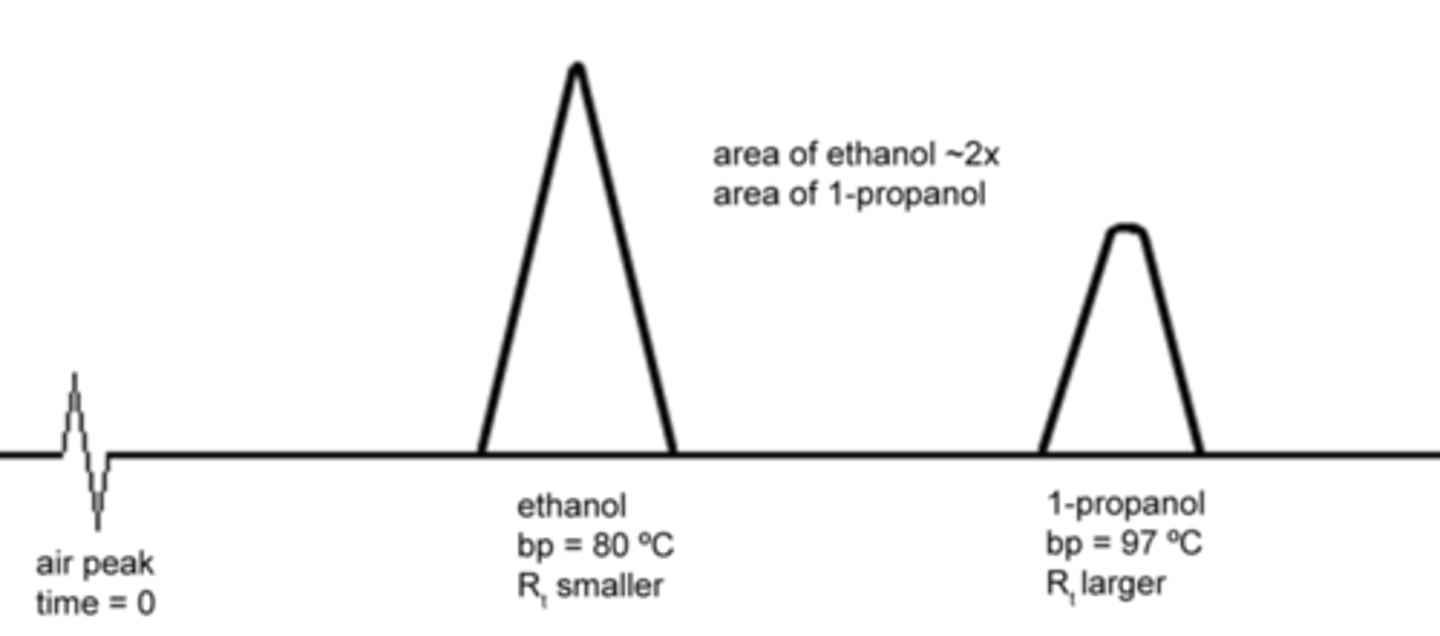
Compound A (MW = 342.8) and compound B (MW = 45.82) have the same boiling points. Which will reach the detector first in Gas Chromatography? Why?
Compound B will reach the detector first because it is smaller and thus will be pushed faster by the Mobile Phase than compound A.
Why is Gas Chromatography often coupled with other procedures such as Mass Spectrometry?
Because Gas Chromatography alone is not necessarily accurate enough in identifying exact compounds.
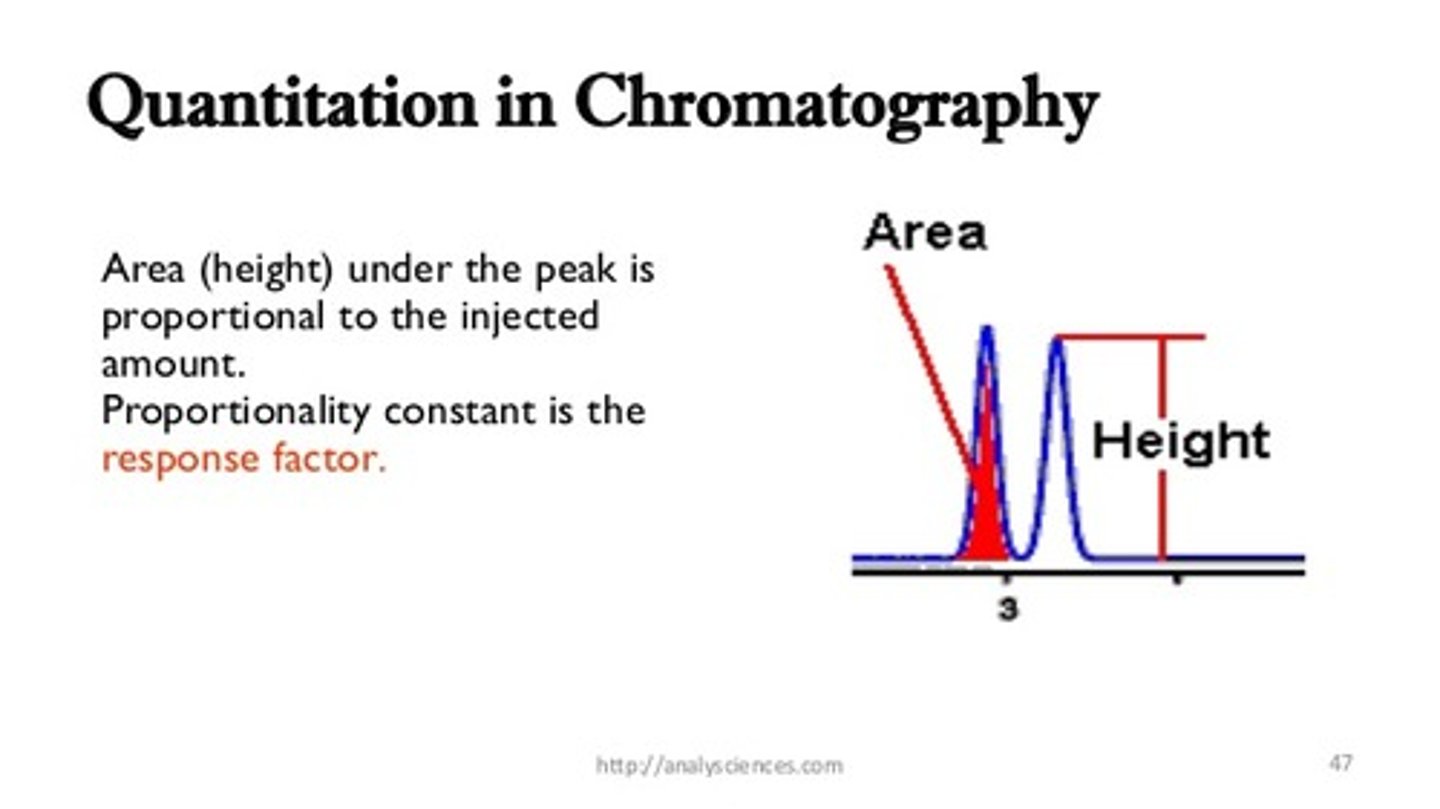
True or False. The area under the curve of each Gas Chromatography peak is directly proportional to the speed at which the compound traveled in the column.
False. The area under the curve of each Gas Chromatography peak is directly proportional to the amount of that compound in the mixture.
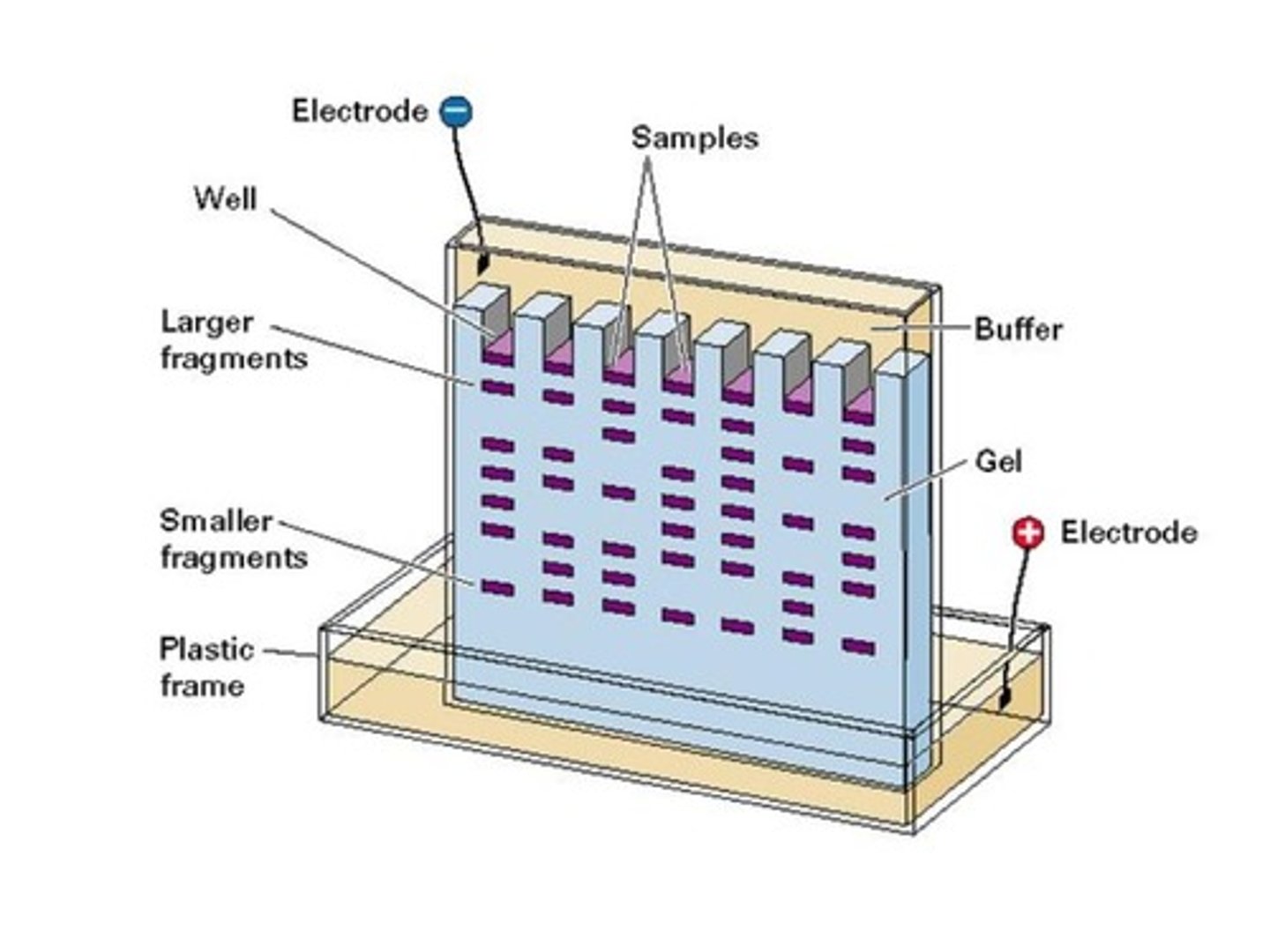
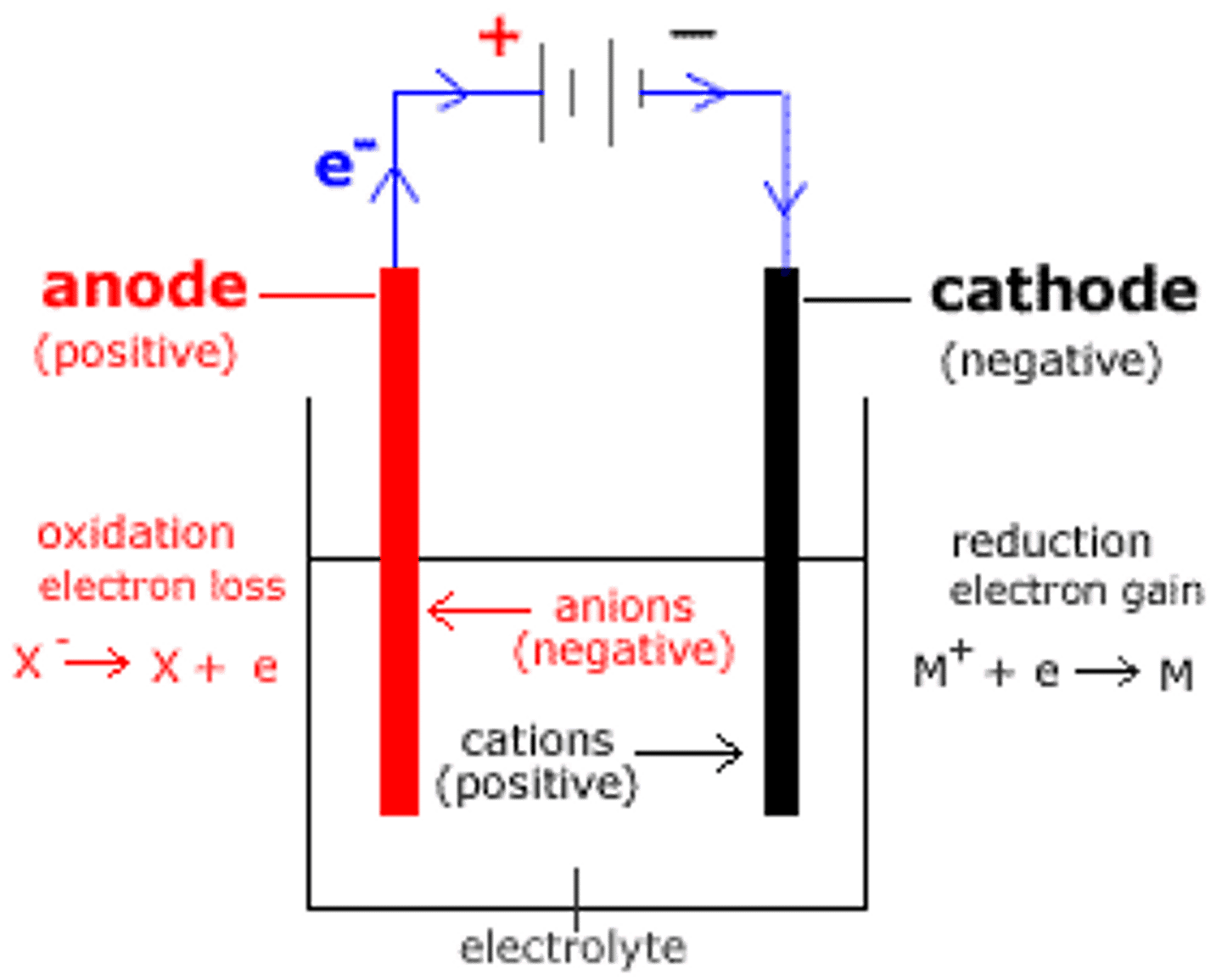
In gel electrophoresis, the cathode produces a ___________ charge while the anode produces a ___________ charge.
(A) negative, positive
(B) positive, neutral
(C) neutral, negative
(D) positive, negative
(A) negative, positive
Reduction takes place at the cathode. Those lost electrons then travel toward the positively charged anode, which is where oxidation takes place.
Which of the following is true concerning DNA Gel Electrophoresis?
(A) Negatively Charged DNA travels toward the Cathode.
(B) Negatively Charged DNA travels toward the Anode.
(C) Positively Charged DNA travels toward the Cathode.
(D) Positively Charged DNA travels toward the Anode.
(B) Negatively Charged DNA travels toward the Anode.
In Gel Electrophoresis, negatively Charged DNA (negative due to the phosphate backbone) will travel toward the positively charged anode.
CRB Does Gel Electrophoresis utilize an electrolytic or a galvanic cell?
Gel Electrophoresis utilizes an electrolytic cell. It is using energy to drive a non-spontaneous reaction (i.e. the movement of the proteins or DNA in the gel).
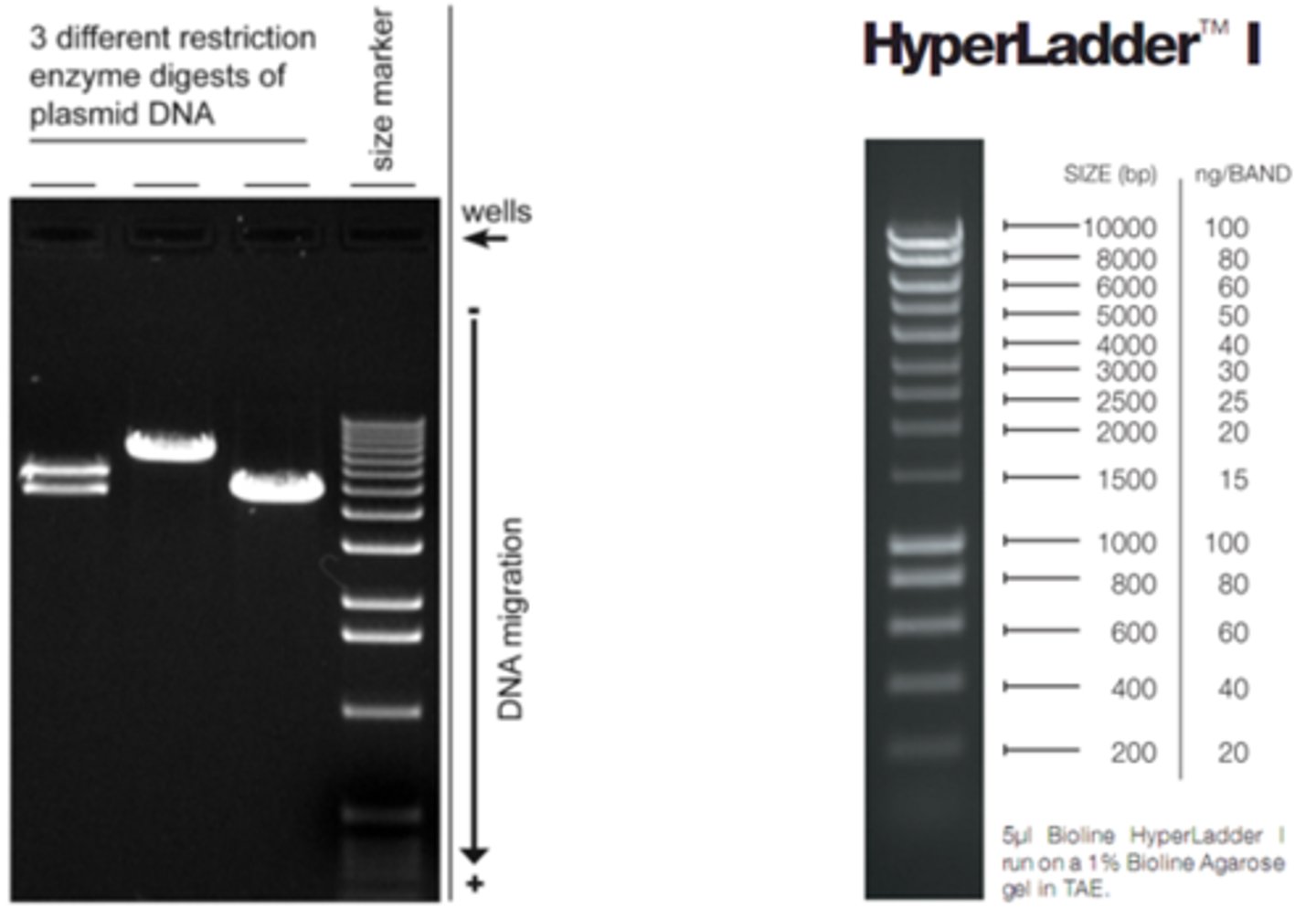
What is the purpose of a DNA Ladder when doing Gel Electrophoresis?
It is a standard that allows you to determine the molecular weights of your compounds.
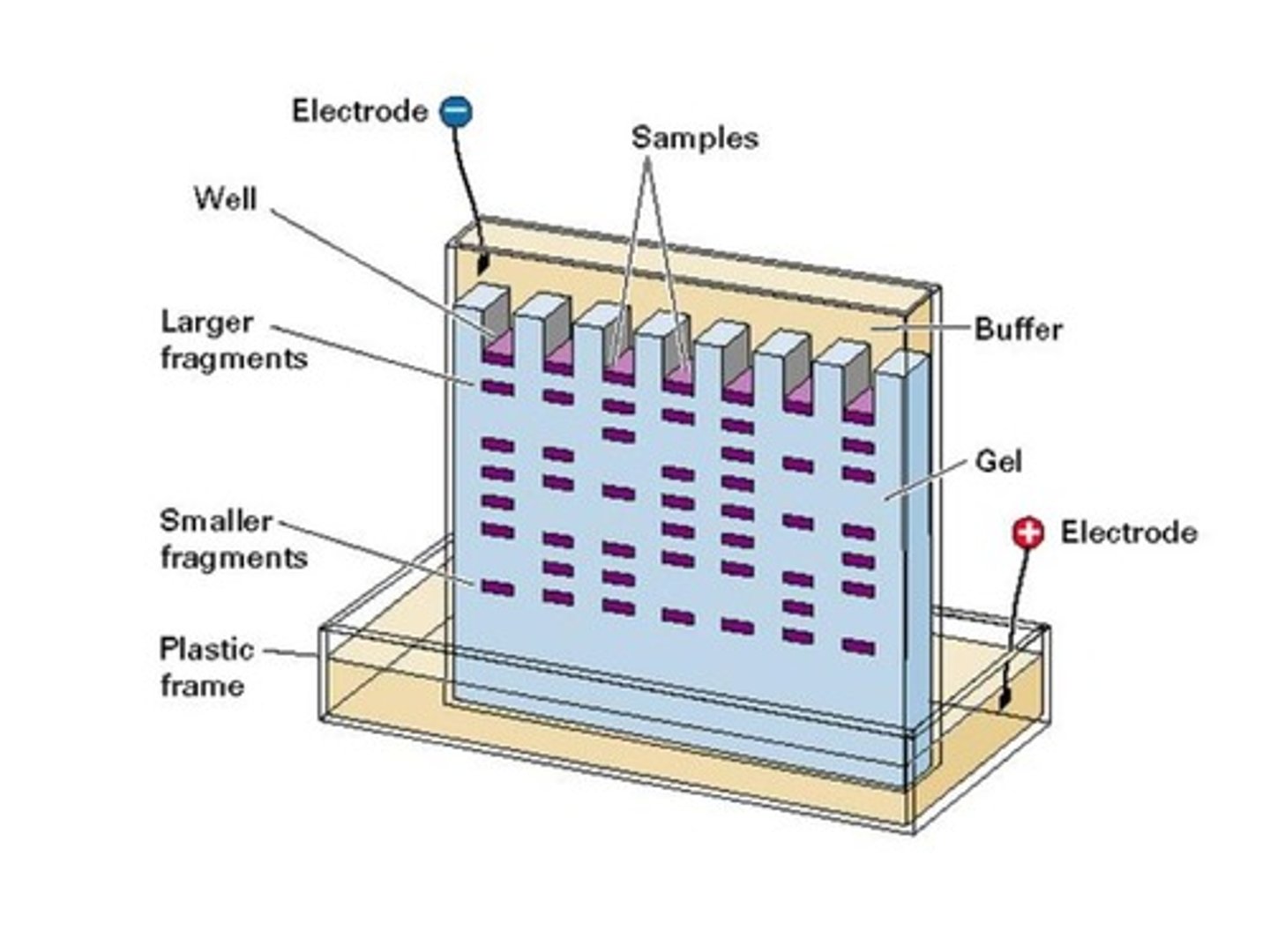
True or False? Large fragments travel the farthest in DNA Gel Electrophoresis because they are more negatively charged than smaller fragments.
False. Small fragments travel farther in DNA Gel Electrophoresis because small fragments experience less resistance as they are traveling through the gel. Large and small fragments have the same mass to charge ratio; therefore, charge is an inconsequential factor.
When is agarose gel used as compared to SDS-PAGE?
Agarose gel is typically used for large compounds, like longer strands of DNA. SDS-PAGE is used for smaller compounds, like shorter strands of DNA (<50bp) and proteins.
What procedure can be used to separate enantiomers from each other?
You do Affinity Chromatography (Column or Gas) in which the Stationary Phase contains a chiral enzyme that only binds to one of the two enantiomers.
CRB Which of the following terms refers to the liquid that is left over after filtrations?
(A) Residue
(B) Filtrate
(C) Solvent
(D) Residual Liquid
(B) Filtrate
The filtrate is the liquid that passes through the filter, and what remains on the filter is the Residue.
CRB Fill in the blanks: In ___________ filtration, the filtrate is often the desired product and you want to minimize any recrystallization. In __________ filtration, the residue is often the desired product.
(A) Vacuum, Gravity
(B) Vacuum, Funnel
(C) Funnel, Gravity
(D) Gravity, Vacuum
(D) Gravity, Vacuum
In Gravity filtration, the filtrate is often the desired product and you want to minimize any recrystallization. In Vacuum filtration, the residue is often the desired product.
Vacuum filtration can trigger recrystallization from using the cold air to suck the filtrate down through the filter paper!