Activity 11 | Molecular Modeling
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Quantum Mechanical (QM) model
Rely on mathematical representations of the electrons and nuclei that make up the molecule
Molecular Mechanical (MM) model
Describe the interactions between the atoms of a molecule using a force field
Valence Shell Electron Pair Repulsion theory (VSEPR)
A model used to predict the geometry of molecular structures based on the idea that electron pairs around a central atom will arrange themselves as far apart as possible to minimize repulsion
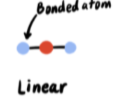
0 lone pairs → 2 electron pairs
Linear (180°)
0 lone pairs → 3 electron pairs
Trigonal planar (120°)

0 lone pairs → 4 electron pairs
Tetrahedral (109.5°)

0 lone pairs → 5 electron pairs
Trigonal bipyramidal (90° and 120°)

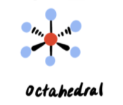
0 lone pairs → 6 electron pairs
Octahedral (90°)
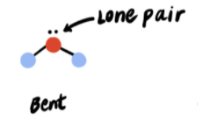
1 lone pair → 3 electron pairs
Bent
1 lone pair → 4 electron pairs
Trigonal pyramidal

1 lone pair → 5 electron pairs
Seesaw

1 lone pair → 6 electron pairs
Square pyramidal

2 lone pairs → 4 electron pairs
Bent

2 lone pairs → 5 electron pairs
T-shaped

2 lone pairs → 6 electron pairs
Square planar

3 lone pairs → 5 electron pairs
Linear

Purpose of experiment
To investigate molecular structures and properties using MolView to visualize geometries and perform energy minimization with the MMFF94 model, while comparing findings to the VSEPR theory predictions