Orgo-Functional Groups
1/32
Earn XP
Description and Tags
suffering or smth idfk
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
What do hydrocarbons contain
only hydrogen and carbon
three types of hydrocarbons
alkanes, alkenes, alkynes
alkanes
only single C-C bonds
alkenes
at least one double C=C bond
alkynes
at least one triple C bond
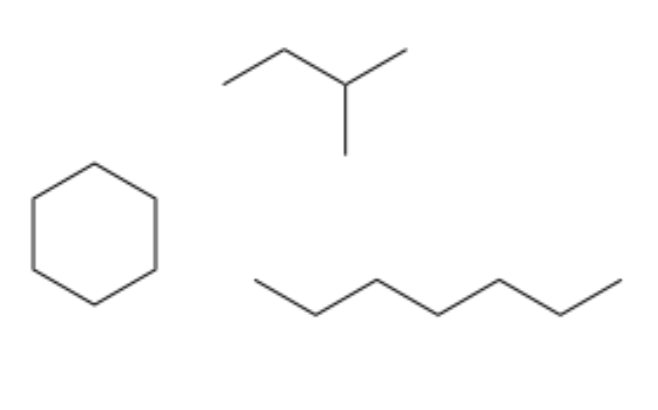
type of hydrocarbon
alkanes
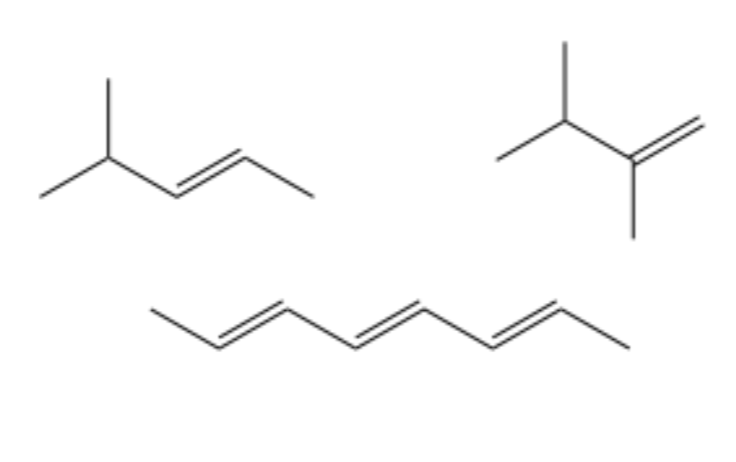
type of hydrocarbon
alkenes
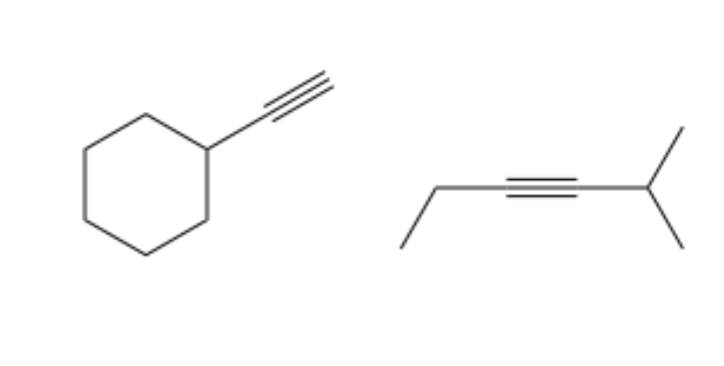
type of hydrocarbon
alkynes
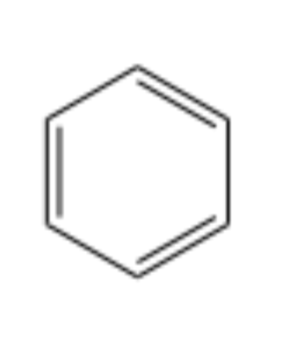
special type of hydrocarbon
benzene
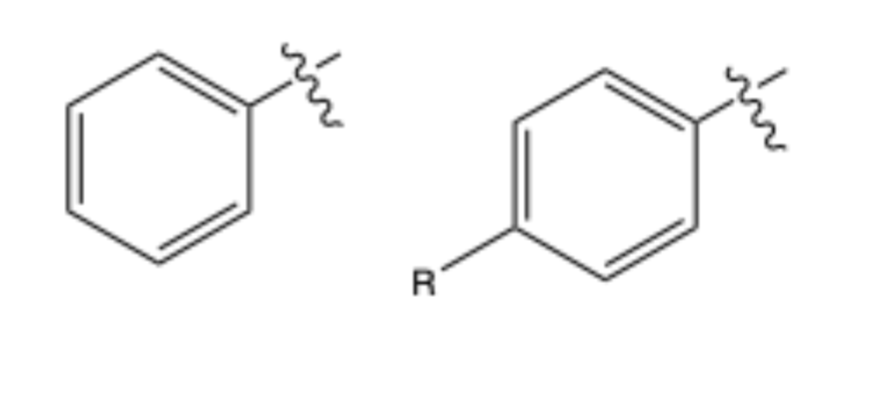
compounds with benzene
aromatic ring or arene
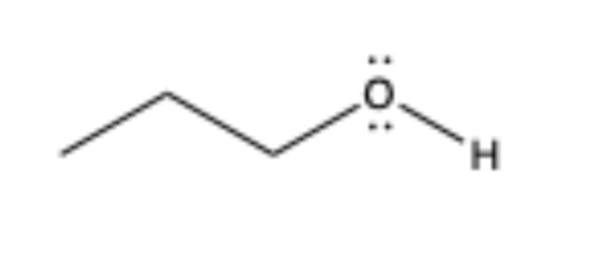
alcohol
contains OH bonded to sp3 C
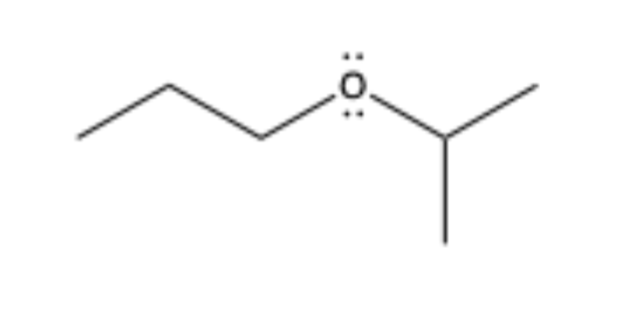
ether
contains O bonded to sp3 C on both sides
alcohol
ether
Carbonyl functional groups
alehydes and ketones
what are carbonyl functional groups
contain a carbonyl bond (C=O)
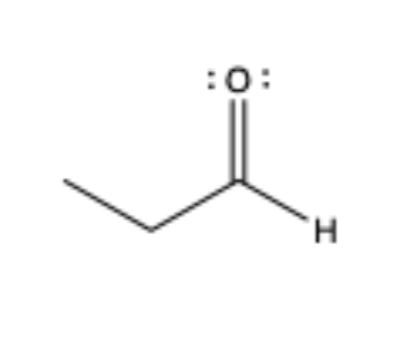
alehyde
has C=O that is bonded to an sp3 C on one side and H atom on the other
ketone
has C=O that is bonded to sp3 C on both sides
aldehyde
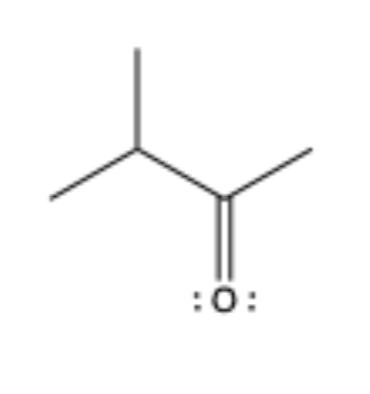
ketone
carboxylic acid
has C=O, C is sp2 not sp3 bonded to OH
ester
contains C=O, sp2 C bonded to O and more Cs/Rs
amide
contains C=O to sp2 C, bonded to N
acid chloride
contains C=O bond to sp2 C, bonded to any halogen (name changes based on halogen)
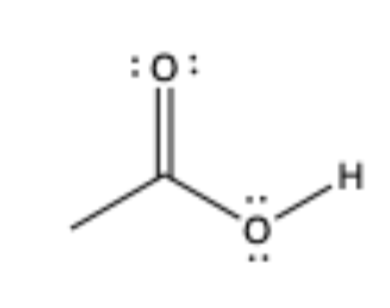
carboxylic acid
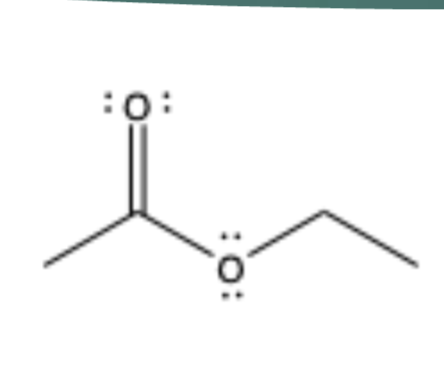
ester
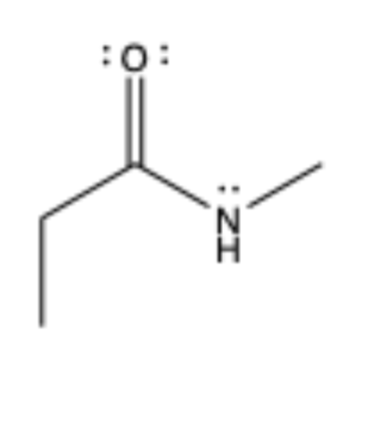
amide
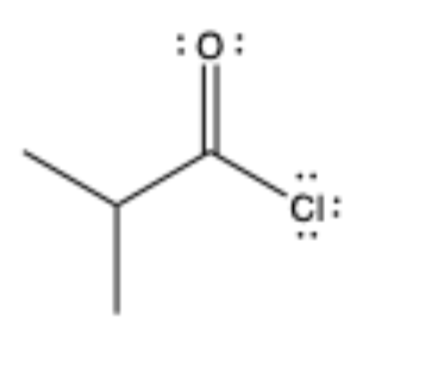
acid chloride
what are nitrogen functional groups
amine, inime, nitrile, and pyridine
amines
N with only three single bond and one lone pair
imine
contains one double bond N
nitrile
contains triple bond N
pyridine
6 membered ring with one N and 3 alt. double bonds