Historical Chemists
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Created the "Greek Atom" Model (Spiky, Swirl, Square)
Democritus (Red)
Claimed that everything was made of particles called "atoms:" (atomos)
Democritus (Red) (1)
Did not provide evidence or experimentation for his claims.
Democritus (Red) (2)
Created the "Solid Sphere" model-- atoms are solid spheres.
John Dalton (Orange) (1)
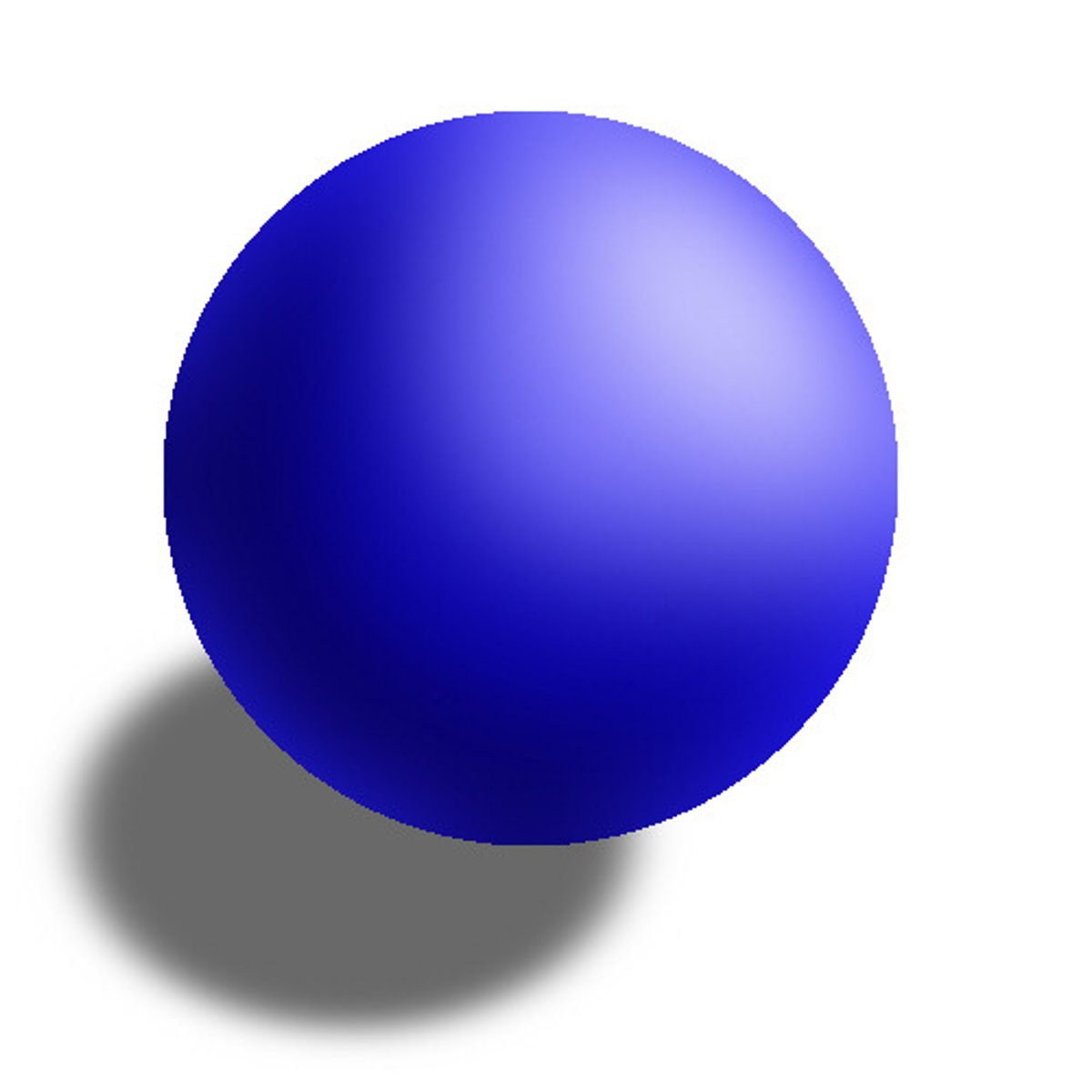
Believed atoms could be "rearranged" like the letters of a word to create elements.
John Dalton (Orange) (😅
Shared the opinion with Democritus that atoms could not be: "split, created, or destroyed."
John Dalton (Orange)
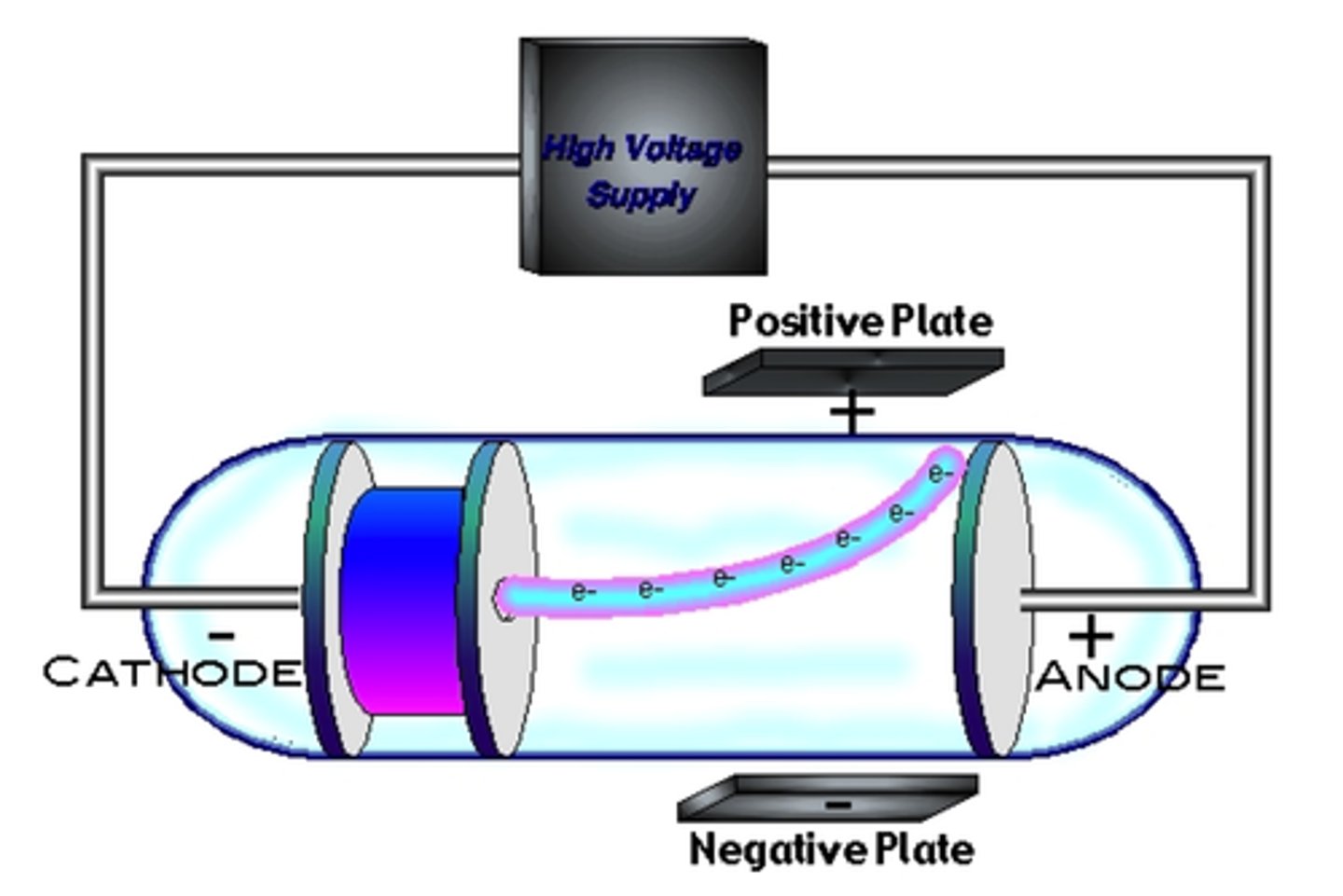
Famous for the Cathode Raytube experiment (CRT)-- a band of light shot through a glass tube and moved toward the positively charged plate, suggesting the particle was negative.
JJ. Thompson (Yellow)
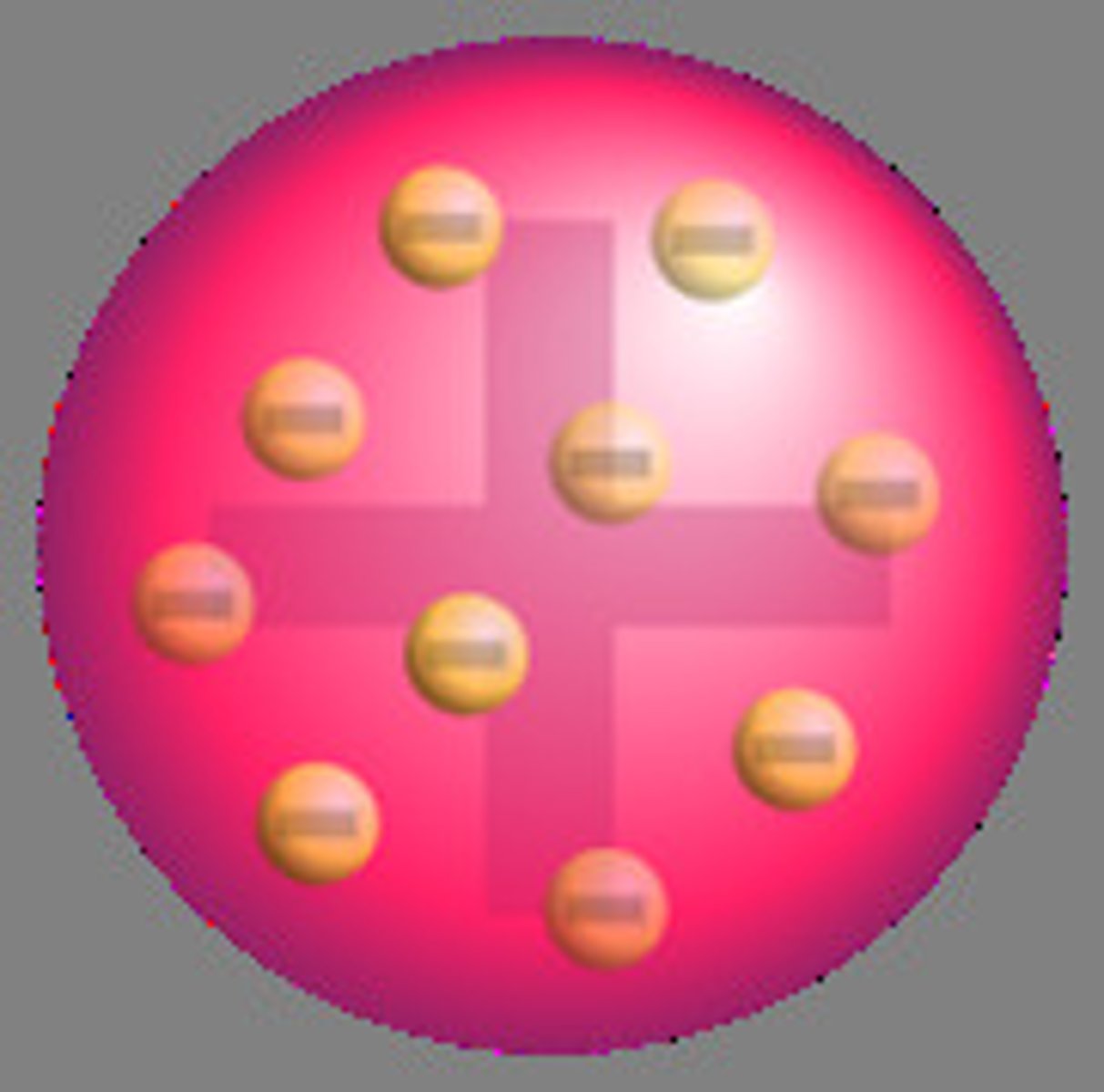
Created the "Plum-Pudding" or "Watermelon" model-- the electrons were suspended in a positively charged 'goop.'
JJ. Thompson (Yellow)
Discovered the Electron, which led to the discovery of the Proton.
JJ. Thompson (Yellow)
Discovered Protons and the Nucleus.
Earnest Rutherford (Green)
Created the "Planetary Model"-- protons bunched in the center and electrons orbiting it-- and disproved the "Plum-Pudding" model.
Earnest Rutherford (Green)
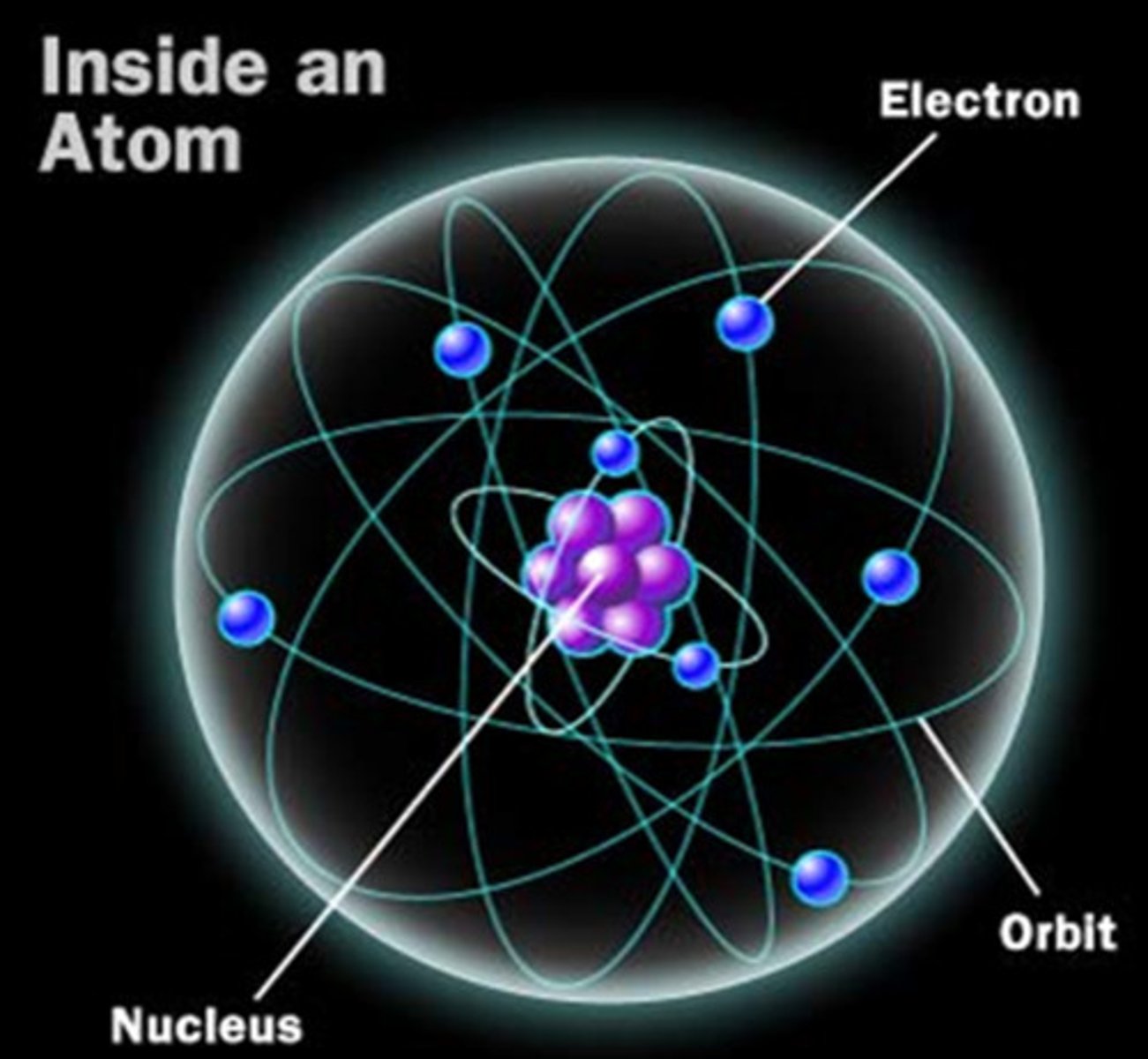
Famous for the "Gold Foil" experiment-- where heavy positively charged alpha particles bounced off gold foil.
Earnest Rutherford (Green)
Made the "Ring" Model-- electrons are in rings that have different energy levels and hold different levels of electrons.
Niels Bohr (Blue)
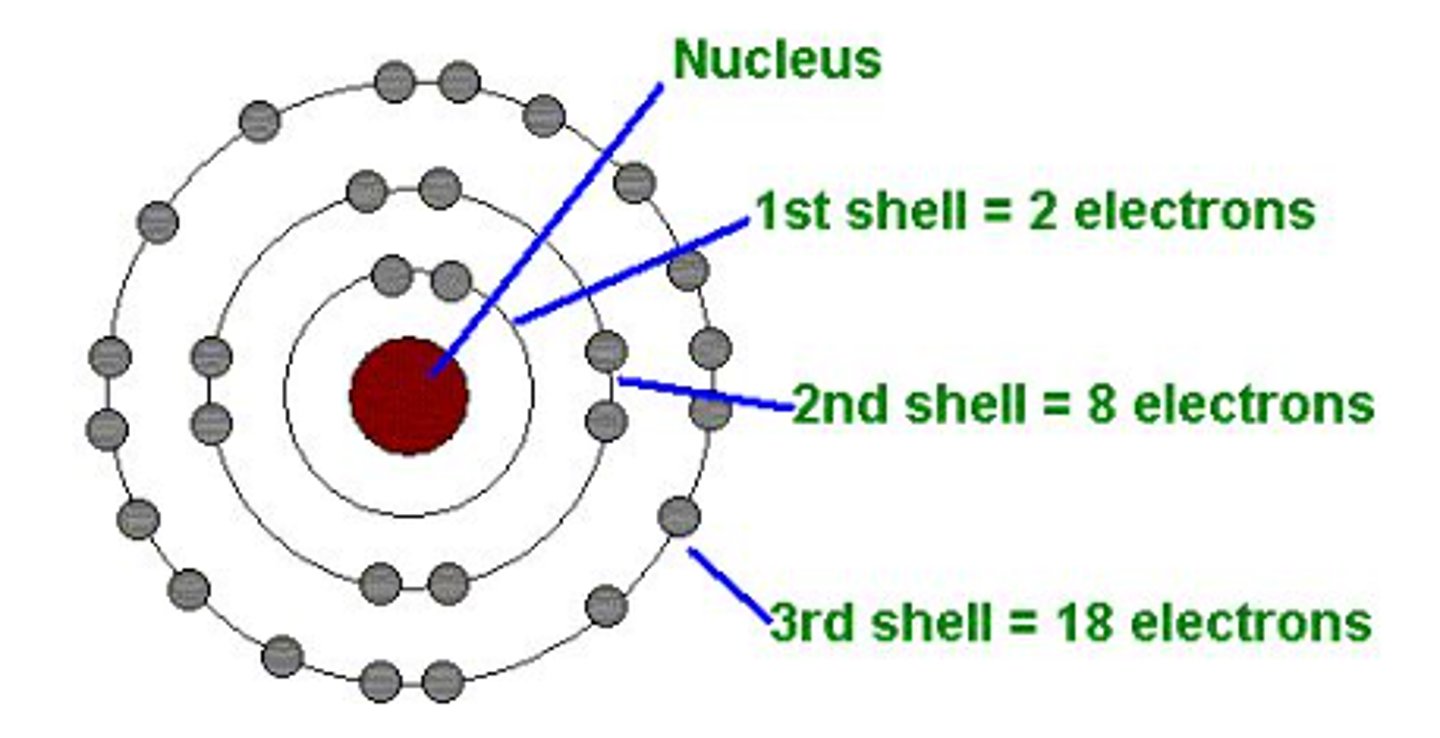
Theorized that nobel gas is stable because they have full outer rings.
Niels Bohr (Blue)
Theorized that Ions are wannabe nobel gases because they gain/lose electrons to have a full outer ring.
Niels Bohr (Blue)
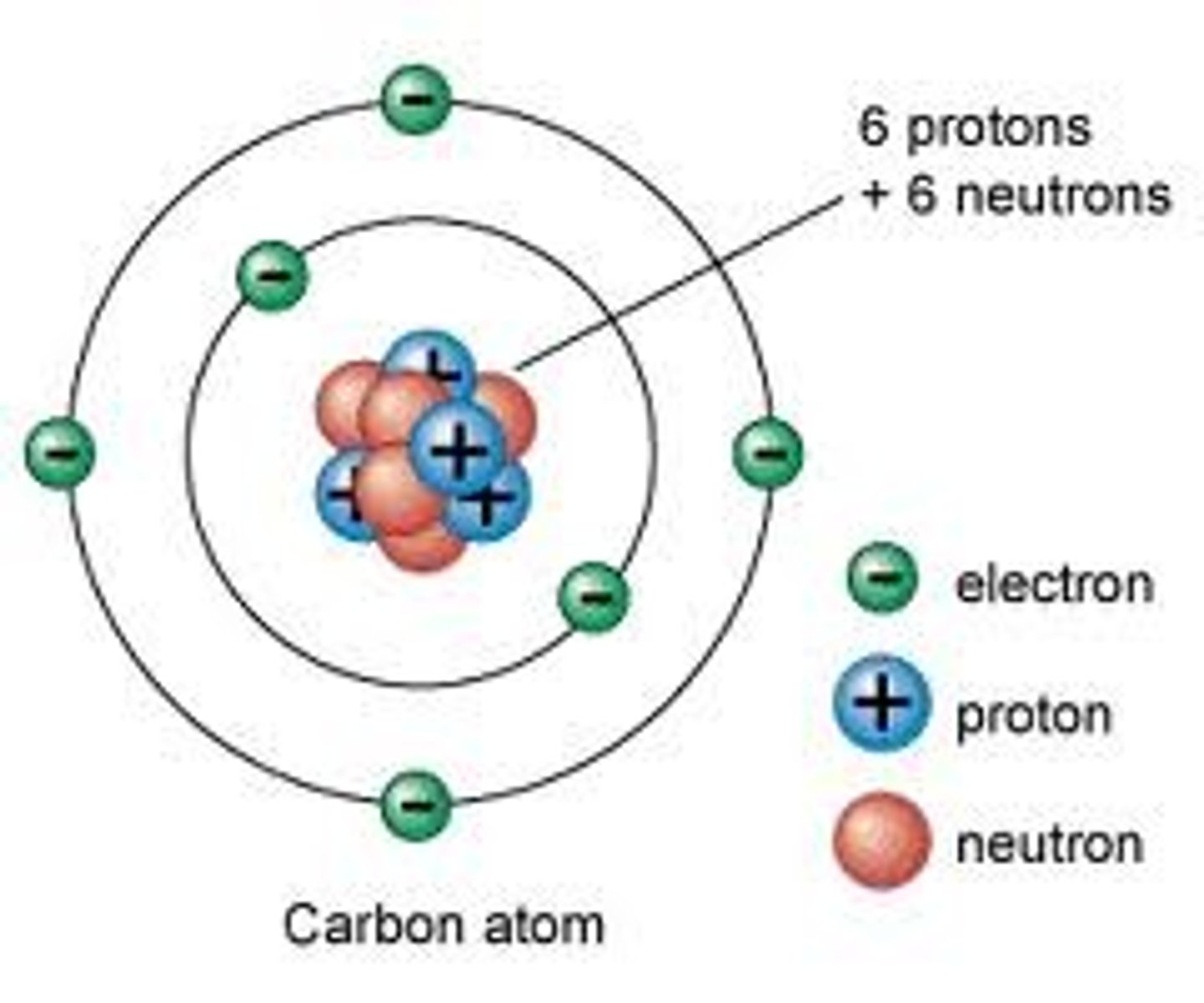
Discovered the Neutron, and found protons and neutrons in the nucleus.
James Chadwick (Purple)
Made the "Neutron Model" which branched off the "Ring Model" but with a fully developed nucleus.
James Chadwick (Purple)
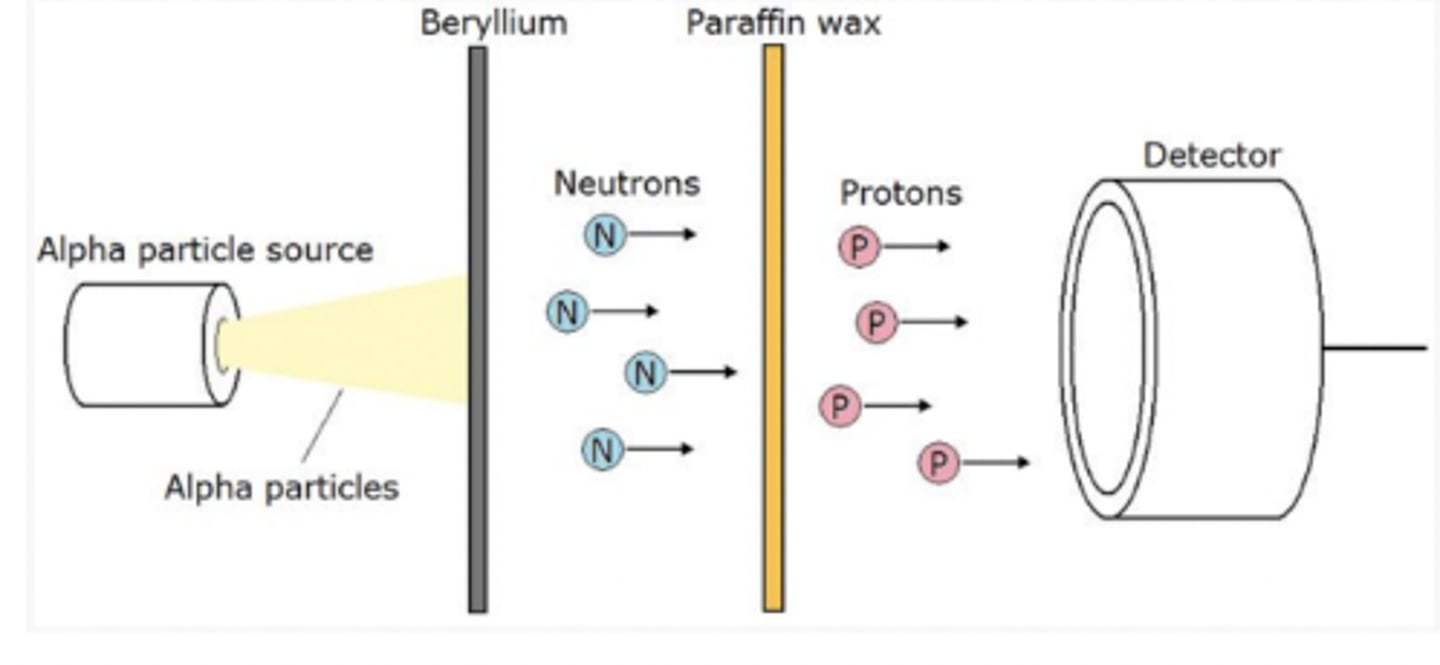
Famous for the "Beryllium Bombardment Experiment" (or "James Chadwick Experiment")-- 4 AMU alpha bullets were shot through Beryllium and positively charged wax paper, and switched into protons; proving it must be neutral.
James Chadwick (Purple)
Father of Quantum Mechanics Theory.
Heisenberg (Pink)