Unit 23 Nuclear Physics
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Define the decay constant.
Probability of decay
of a nucleus per unit time
Suggest why dust that has been contaminated with Strontium-90 presents a serious health hazard.
small volume of strontium-90 has high activity
can be breathed in easily so presents health hazard
e- + e+ → 2 γ
Assuming that the kinetic energy of the positron and the electron is negligible when they interact, suggest why the two photons will move off in opposite directions with equal energies.
momenta must be equal and opposite after
since momentum before is zero
equal momentum after so momenta of photon equal and in opposite directions so photon energies equal
A radioactive element has a half-life of approx. 4 hours.
Suggest why measurement of the mass and activity of a sample of this element is not appropriate for the determination of its half-life.
Sample would decay appreciably whilst measurements are being made.
State what is meant by nuclear fission
Large nucleus splits
into two nuclei of approximately equal mass
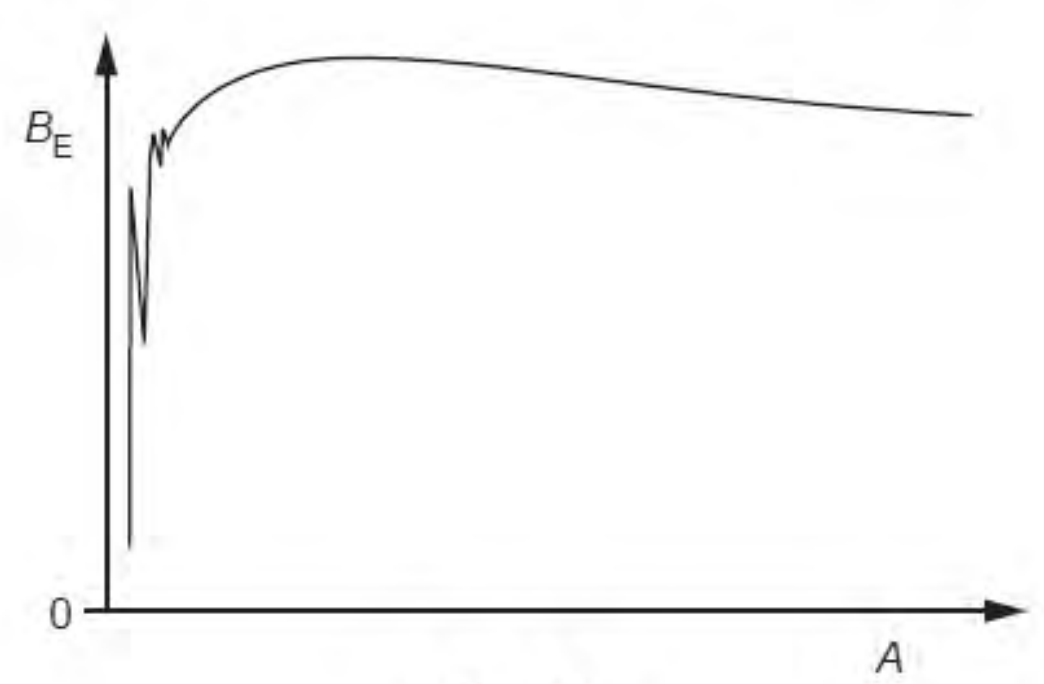
By reference to the figure, explain how fission is energetically possible.
binding energy = BE × A
binding energy of parent nucleus is less than sum of binding energy of the fragments