Basics of Haloalkanes
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What is a haloalkane
A saturated organic compound containing one or more halogen substituents bonded to a sp3 hybridised carbon atom.
What elimination and substitution reactions do they undergo.
Readily undergo substitution reactions with nucleophiles
Undergo elimination reaction with bases to give an alkene involving the loss HX.
How are haloalkanes classed
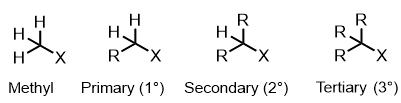
What is a substitution reaction
When a substituent in a molecule is replaced with another
What are the two ways a substitution reaction may occur in haloalkanes
Nucleophilic attack at the same time as the leaving group leaving the molecule (Sn2 mechanism)
Leaving group leaves first to form a carbocation intermediate that is then attacked by a nucleophile in a second step (Sn1 mechanism)
What’s an elimination reaction
Where a substituent group is removed from the molecule
What are the two types of elimination reactions occuring in haloalkanes
Deprotonation at the same time as the leaving group leaving (E2 mechanism)
Leaving group leaves first to form a carbocation intermediate that is then deprotonated by a base in a second step (E1 mechanism)
What’s a Leaving group
Refers to what group is either substituted or eliminated
How does the leaving group effects rate of reaction
Good leaving groups make reactions faster, poor leaving groups slow the reactions down or stop it from happening altogether
Relationship between the leaving group and pKa
a good leaving group is capable of stabilising a negative charge through high electronegativity or by delocalisation of electrons. Low pKa
a poor leaving group won’t be able to do so due to lower electronegativity and more dense electron distribution. High pKa