AQA A level Chemistry 3.1.11 Electrode potentials
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
66 Terms
What happens when a piece of metal is dipped into a solution of its metal ions? (2)
- An equilibrium is established between the metal atoms and its ions in the solution
- A half cell
Draw a diagram to show a half cell set up for zinc (2)
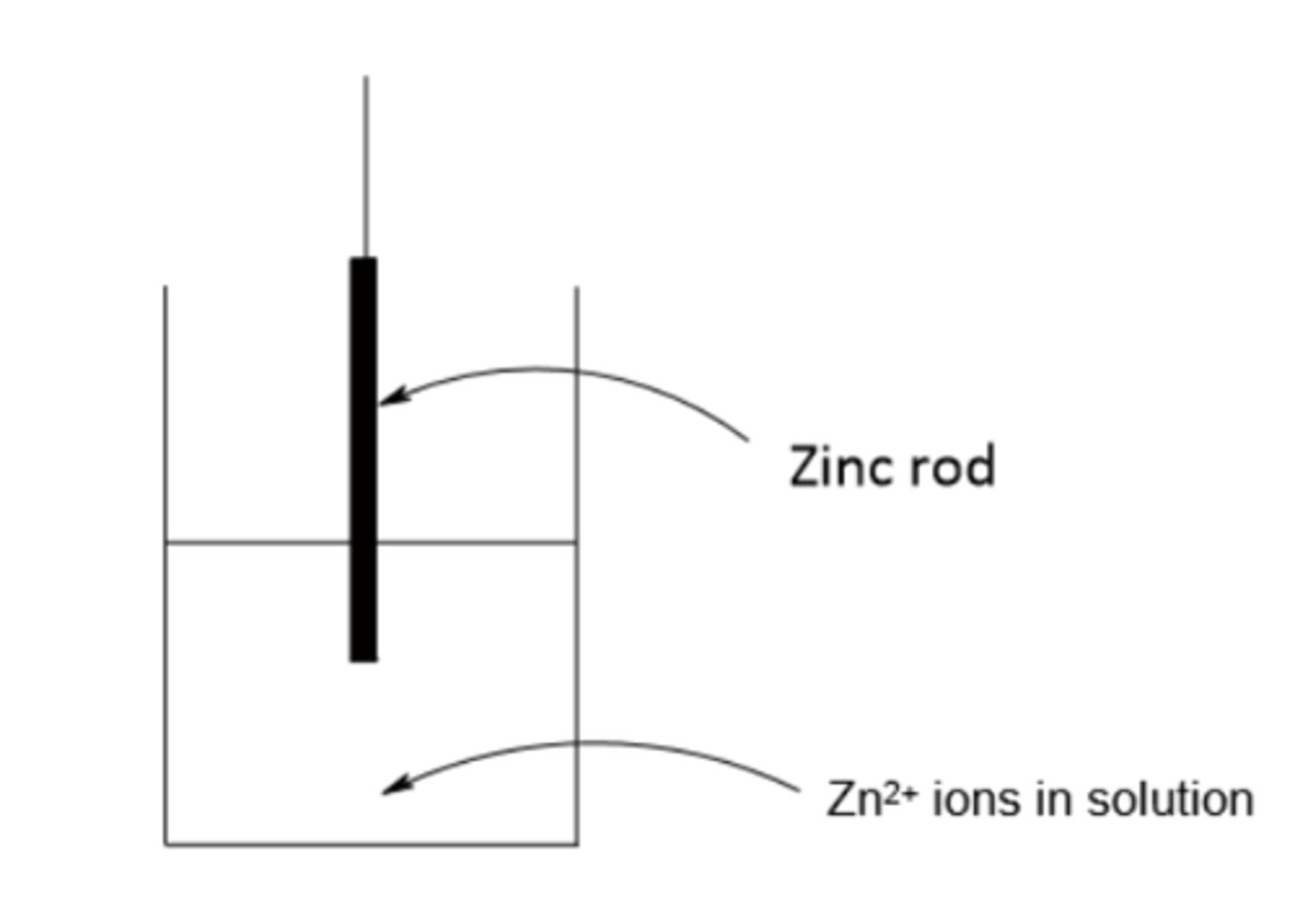
Write an equilibrium reaction for zinc (2)
Zn2+ + 2e- ⇌ Zn
What is the electrode potential (E°)? (2)
- The potential difference set up between the rod and the solution
- Measures how readily electrons are released by the metal.
Draw and label a basic diagram of an electrochemical cell (4)
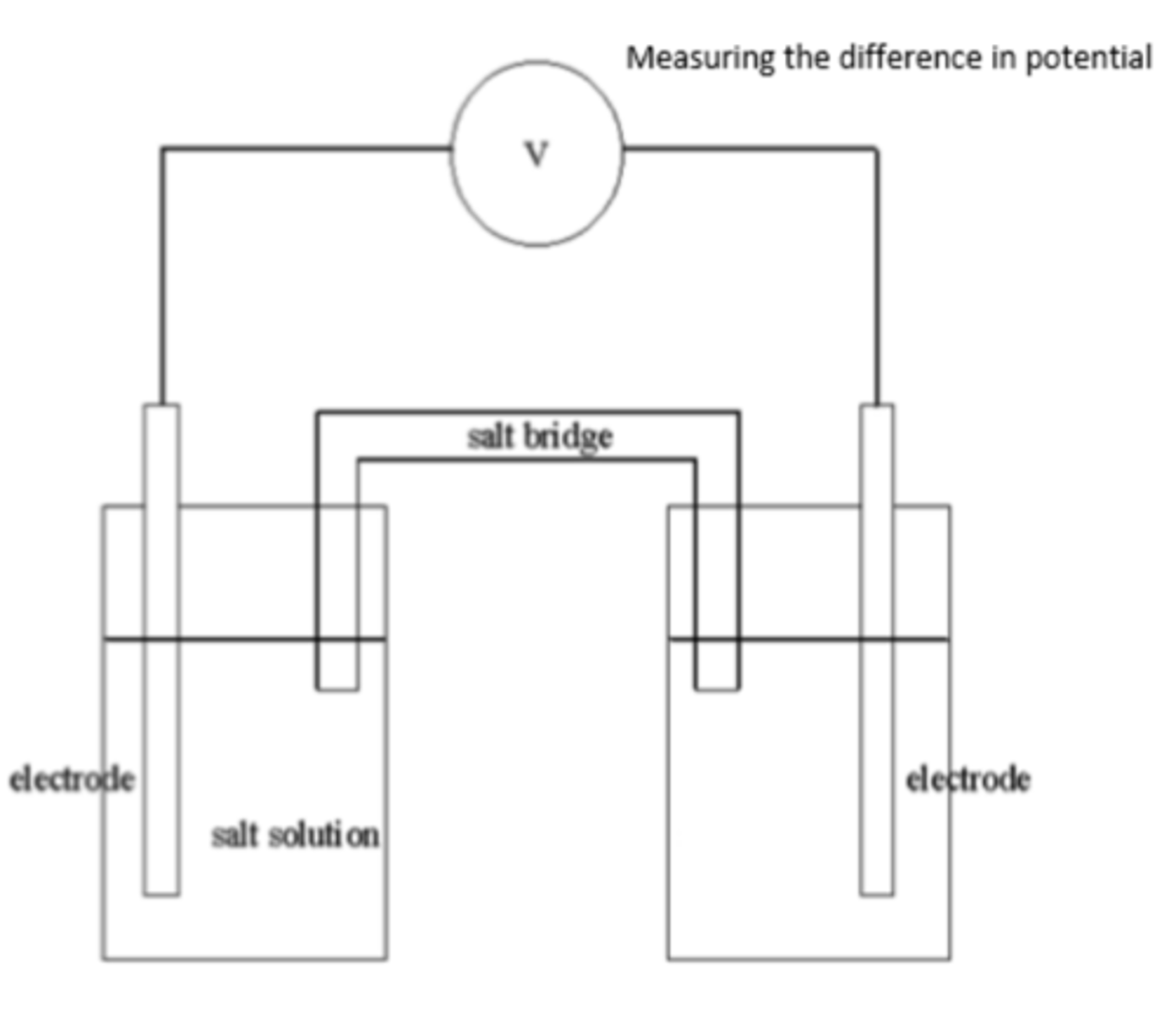
What does a voltmeter measure in an electrochemical cell? (1)
Measures the potential pushing power of electrons through the circuit while keeping the current at zero.
What is the function of the wire in an electrochemical cell? (1)
Allows the movement of electrons.
What are electrodes, and what is their role in an electrochemical cell? (1)
Electrodes are where the half-equations take place, also referred to as the half-cells.
What is a salt bridge? (1)
A filter paper soaked in soloution of KNO3
What is the purpose of the salt bridge in an electrochemical cell? (2)
- Allows the movement of ions to complete the circuit.
- Compensates for changes in ion concentration in each half-cell.
Why is KNO₃ a suitable solution for the salt bridge? (1)
It does not react with any of the ions in solution, so it does not interfere with the redox reaction.
Why is KCl not used as a salt bridge solution for a cell containing Ag⁺ ions? (1)
KCl contains Cl⁻, which would react with Ag⁺ to form a white precipitate.
What are the three types of electrodes? (3)

Why is a standard electrode potential used? (1)
To compare the tendency of different metals to release electrons and determine which electrode will be positive or negative.
What is the role of the Standard Hydrogen Electrode (SHE)? (1)
It is the primary standard assigned a potential of 0 volts as a reference.
Draw the standard hydrogen electrode? (3)
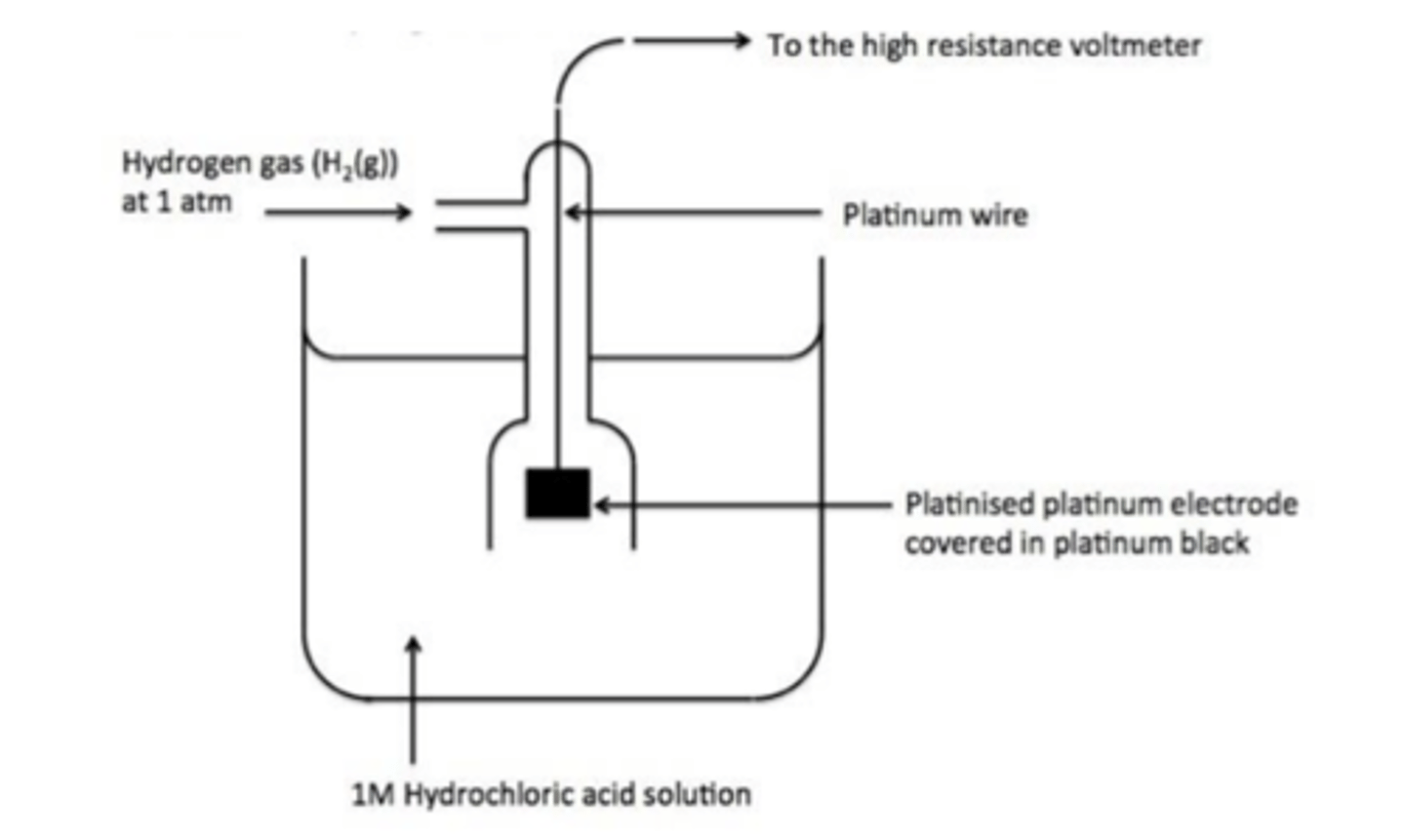
What is the half-equation for the Standard Hydrogen Electrode? (1)
H⁺(aq) + e⁻ ⇌ ½H₂(g)
What is the E° value of the Standard Hydrogen Electrode? (1)
0.00 V
What are the standard conditions for the Standard Hydrogen Electrode? (3)
- 1.00 mol dm⁻³ HCl (pH = 0)
- 100 kPa pressure
- 298 K temperature
What are the components of the Standard Hydrogen Electrode? (3)
- 1.00 mol dm⁻³ HCl
- H₂ gas
- Platinum electrode coated in platinum black
What is the checklist for drawing half cells? (3)

What is an electrochemical series? (1)
The electrode potentials of different ions after they have been measured using the SHE
What are the best reducing agents in terms of electrochemical series? (1)
Very negative potentials
What are the best oxidising agents in terms of electrochemical series? (1)
Very positive potentials
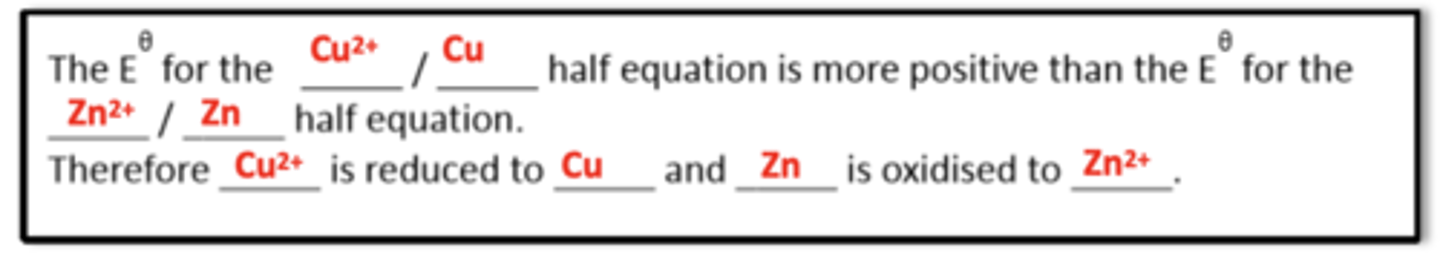
What template answer do you use to explain why a reaction is feasible using electrode potentials? (3)
What is the E∘ cell also known as? (1)
Electromotive force (potential difference) across two electrodes
What does a positive E∘ cell value indicate about the reaction? (1)
The reaction is feasible
What does a negative E∘ cell value indicate about the reaction? (1)
The reaction is not feasible
What is the formula for calculating E∘ i.e. EMF of a cell? (1)

What does a more positive E∘ value indicate about a reaction? (1)
The more positive the value, the more feasible the reaction
Give an example of a conventional cell diagram (1)
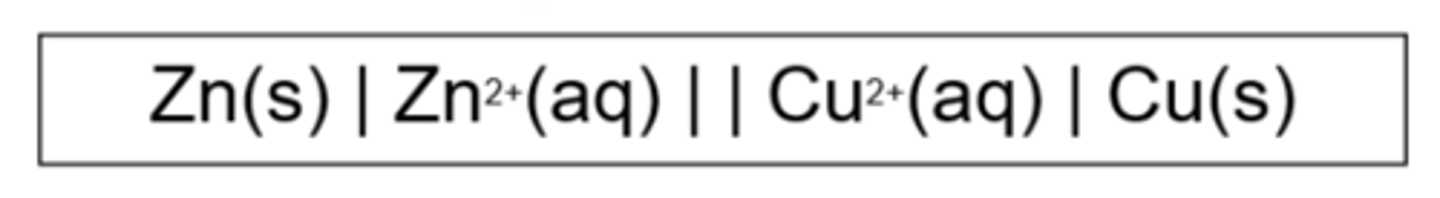
What do single lines (|) in a cell diagram represent?
Phase boundaries i.e. different states
What do commas (,) in a cell diagram indicate?
Commas are used if the phases of the components are in the same phase or are liquid and aqueous
What does a double line (||) in a cell diagram represent?
Salt bridge
Which species is placed on the LHS of a cell diagram?
The species being oxidised (negative electrode)
Which species is placed on the RHS of a cell diagram?
The species being reduced (positive electrode)
When are H+ and H2O included in a cell diagram? (1)
They are included if they are the species undergoing oxidation or reduction
How is a platinum electrode represented in a cell diagram when no solid is present? (1)
Pt
How is the order of elements determined in a cell diagram?
It is based on the direction of their half-equations
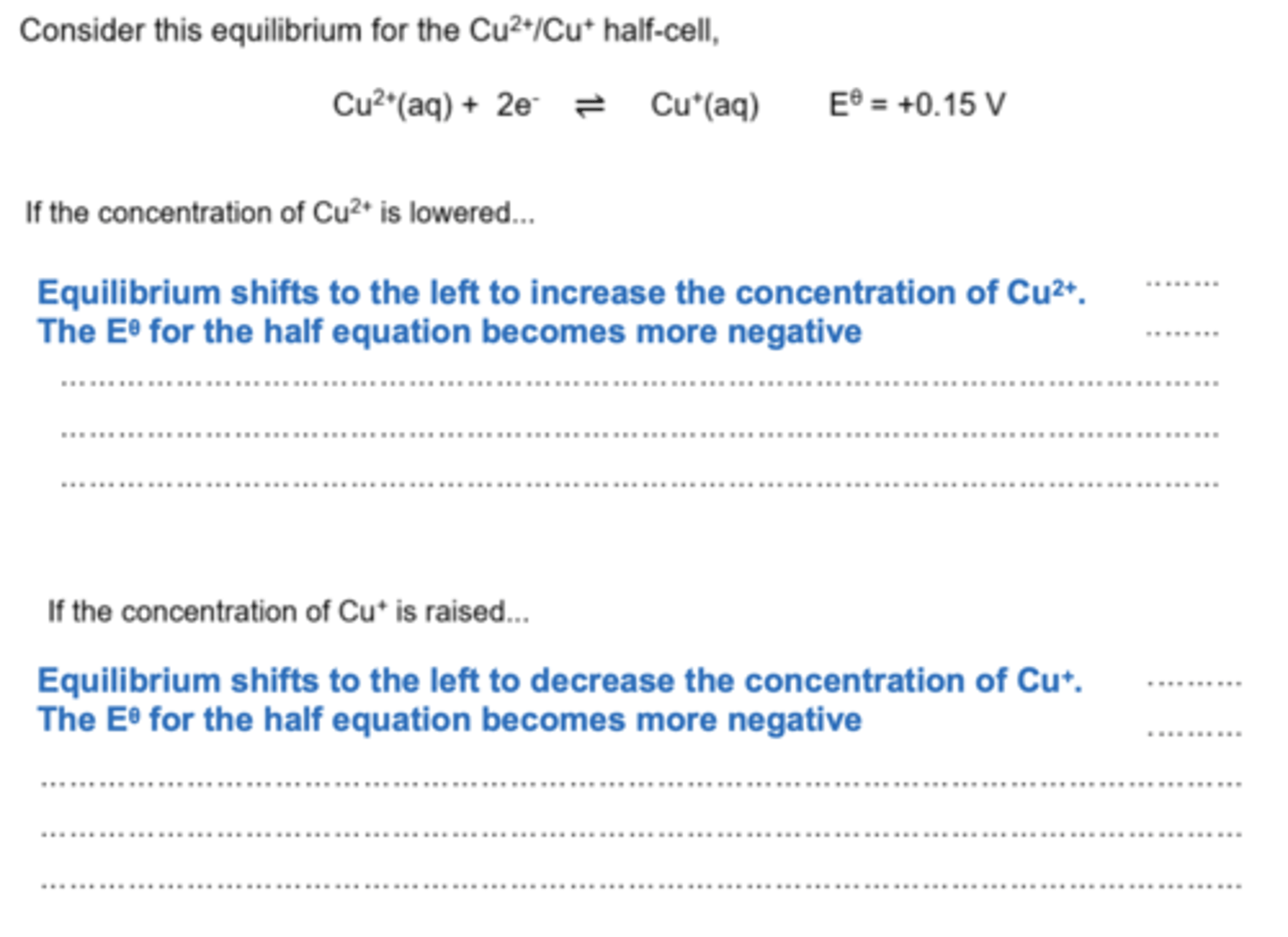
How does shifting equilibrium in a redox half-equation affect the Eo value? (2)
- A shift to the right (oxidation direction) makes the Eo more positive or less negative (depending on sign of Eo).
- A shift to the left (reduction direction) makes the Eo more negative or less positive (depending on sign of Eo)
Example is given
What are examples of non-rechargeable cells? (3)
Zinc-carbon cells, Daniell cell and alkaline cells
What is the Daniell cell? (1)
The Daniell cell uses zinc as the negative electrode and copper as the positive electrode
Why is the Daniell cell non-rechargeable? (2)
- The zinc electrode is consumed over time
- Making the reaction irreversible and impractical for transport
What is the overall reaction during discharge for an alkaline battery? (1)
2MnO2 + 2H2O + Zn → 2MnO(OH) + 2OH- + Zn2+
What is the cathode material in an alkaline battery? (1)
A mixture of graphite and MnO2
What is the anode material in an alkaline battery? (1)
Paste of zinc and KOH
What separates the electrodes in an alkaline battery? (1)
A porous separator soaked in KOH.
What is the overall reaction during discharge for a zinc-carbon cell? (1)
2MnO2 + 2H+ + Zn + 2NH3 → Mn2O3 + H2O + [Zn(NH3)2]2+
Why does the EMF of a zinc-carbon cell decrease over time? (1)
The EMF decreases as the reactants are consumed
What is the reaction at the positive electrode in a lithium cell? (1)
Li⁺ + CoO₂ + e⁻ → Li⁺[CoO₂]⁻
What are examples of rechargeable cells? (3)
- Lithium-ion
- Nickel-cadmium
- Lead-acid cells
What is the reaction at the negative electrode in a lithium cell? (1)
Li → Li⁺ + e⁻
What are the benefits of using cells? (1)
Portable source of electrical energy.
What is the overall reaction during discharge for a lithium-ion battery? (1)
CoO2 + Li → LiCoO2
What is the overall reaction during recharge for a lithium-ion battery? (1)
LiCoO2 → CoO2 + Li
What is the overall reaction during discharge for a nickel-cadmium battery? (1)
NiO(OH) + 2H2O + Cd → Ni(OH)2 + Cd(OH)2.
What is the overall reaction during recharge for a nickel-cadmium battery? (1)
Ni(OH)2 + Cd(OH)2 → NiO(OH) + 2H2O + Cd.
What is the overall reaction during discharge for a lead-acid battery? (1)
PbO2 + Pb + 2H2SO4 → 2PbSO4 + 2H2O.
What is the overall reaction during recharge for a lead-acid battery? (1)
2PbSO4 + 2H2O → PbO2 + Pb + 2H2SO4
What are the risks of using cells? (1)
Waste issues
What are the benefits of using non-rechargeable cells? (1)
Cheap
What are the risks of using non-rechargeable cells? (1)
Waste issues
What are the benefits of using rechargeable cells? (3)
- Less waste.
- Cheaper in the long run.
- Lower environmental impact.
What are the risks of using rechargeable cells? (1)
Some waste issues (at end of useful life)
What are the benefits of using hydrogen fuel cells? (3)
- Only waste product is water.
- Does not need recharging.
- Very efficient.
What are the risks of using hydrogen fuel cells? (4)
- Need constant supply of fuels.
- Hydrogen is flammable and explosive.
- Hydrogen usually made using fossil fuels.
- High cost of fuel cells.