rates of reactions
1/20
Earn XP
Description and Tags
chem
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
tell 6 ways of imcreasing rate of reactions?
increasing concentration
increasing surafce area
increasing pressure
use of catalyst
increasing temp
why greater surface area increases rate?
becuz the greater the surface narea the more the solid is exposed to the liquid
what is differnece between using a loose cotton woll and a wood cap as cap?
cotton wool will prevent liquid from sprayimg outside but will allow ANY GAS to escape however wood cap blocks both
what part of the graph shows that a reaction has a fatser rate?
the slope would be steeper in graph
what is one thing that doesnt change when u increase the rate of reaction?
the amount of product formed will be the same as on graph they will connect
why does pressure incrtease rate of reaction?
the gas partricles are pushed together and collide /react more readily ,increasing the rate of reaction.
why does temp affect the rate of reaction?
cuz the rate of decay and oxidation by air is slower at low temp
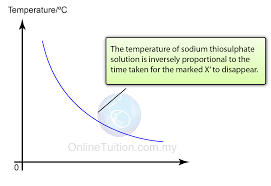
what is the inverse relation graph?
give imp catalyst name?
maganese oxide powdered
what is a catalyst tell in detail?
increases rate of chemical reaction
it renmains unchanged during reaction
can be reused
which group is better at being catalkyst or forming cataytic compounds?
transition elements
what are enzymes?
protein molecu;es that act as biological catalysts
hwo to differ that which reaction is reacting fatsrer?
change in colour
change in conductivity
change in ph
elements involving gas can be found out by measuring the gas using gas syringe
when evaluating experiments what should u think abpout?
clean apparatus
accuarcy of measuring apparatus
number iof times experiment can be recreated to make sure results obtained are consistenet
hpow easy it is to control varibales sucgh as heat etc
what is collision theory?
a theory which states that a chemical reaction takes place when particles of the reactants collide with sufficent energy to initiate reaction.
collsison theory in temp increase?
Particles gain more kinetic energy.
They move faster, causing more frequent collisions.
A greater proportion of particles have energy equal to or greater than the activation energy.
Therefore, the frequency of successful collisions increases, making the rate of reaction faster.
collsison theory when pressufre imncreases?
The particles are closer together, meaning the same number of particles occupy a smaller volume.
This results in more frequent collisions between gas molecules.
Therefore, the frequency of successful collisions increases, speeding up the rate of reaction.
incteasing concenmtration?
There are more reacting particles per unit volume.
This causes more frequent collisions between reactant particles.
Hence, the number of successful collisions per second increases, and the rate of reaction increases.
increasing surface area?
When the surface area of a solid reactant is increased (for example, by using smaller pieces or powder):
More particles are exposed to the other reactant.
This gives a larger surface for collisions to occur.
Therefore, the frequency of collisions between reacting particles increases.
This leads to more successful collisions per second, increasing the rate of reaction.
why ativation energy is imp?
each reaction has its own value of activatrion energy ,as energy is needed to break bonds in reactant molecules,and when tge partlce collide they must have energy graeter than activation energy
what does catalyst do ?
reuces activation energy or energy needed to break bonds