Chapters 8, 9, 10: Reactions of Alkenes, Alkynes, Alcohol Synthesis
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
1) Addition of Hydrogen Halides
HBr
Markonikov
2) Free Radical Addition of HBr
HBr, H2O2
Anti-Markonikov
3) Hydration of Alkenes
H3O+
Markonikov, rearragement
4) Oxymercuraiton-Demurcuraiton
1) Hg(OAc)2 , H2O 2) NaBH4
Markonikov, ANTI
5) Hydroboration
1) BH3-THF 2)H2O2, NaOH
Anti-Markonikov, syn
6) Addition of Halogens
X2
ANTI
7) Formation of Halohydrins
X2 + OH
Markonikov, anti
8) Catalytic hydrogentation
H2, Pt/Pb/Ni
Syn
9) Epoxidation
Peroxyacid
Retains stereochemistry
10) Acid-catalyzed opening of epoxides
H3O+
Anti diols
11) Syn dxhydrogenation of alkenes
a) OsO4, H2O2
b) KMnO4, cold, dilute
12) Oxidative cleavage of alkenes
a) KMnO4, warm, concentrated
Makes carboxylic acid
b) Ozonolysis (O3, (CH3)2S
Makes aldehydes
1) Formation of Acetylide Ions
Aklyne + NaNH2
1) A. Synthesis of Alkynes From Acetylide
SN2 attack
1) B. Addition of Acetylide to Carbonyl Groups
Nucleophliic addition
2) Synthesis of Alkenes by Elimination Reactions
A) Dehalohydrogentation
Two moles of HX
B) KOH at 200ºC to give internal alkynes
C) 1) NaNH2 at 150ºC 2) H2O to give terminal alkynes
3) Addition Reactions of Alkynes 1
A) Catalytic Hydrogenation
B) Partial Hydrogenation to cis alkenes
C) Metal-Ammonia Reduction to trans alkenes
D) Addition of Halogens
E) Addition of Hydrogen Halides
AntiMARK by adding ROOR
F) Hydration of Alkynes to Ketones and Aldehydes
Mercuric Ion-Catalyzed Hydration
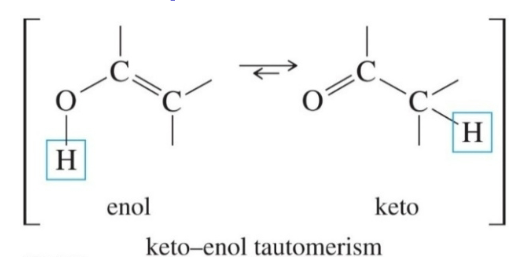
4) Keto-Enol Tautomerism
5) Hydroboration Oxidation
1) Sia2BH 2) NaOH, H2O2
Anti-Markonikov
6) Oxidation of Alkynes
A) Permanganate oxidation
B) Ozonolysis
3) A. Catalytic Hydrogenation
Addition of two moles of H2 w/ Pt/Pd/Ni
3) B. Partial Hydrogenation
One mole of H2 and Pt/Pd/Ni to give cis alkene
3) C. Metal-Ammonia Reduction
Addition of NaNH2 to give trans alkenes
3) D. Addition of Halogens
1 mole of X2: Forms vinyl dihalides (both cis and trans isomers)
2 moles of X2: Forms tetrahalides
3) E. Addition of Hydrogen Halides
Markonikov
1 mole HX: vinyl halides
2 mole HX: Geminal dihalide
AntiMARK:
Add peroxides (ROOR)
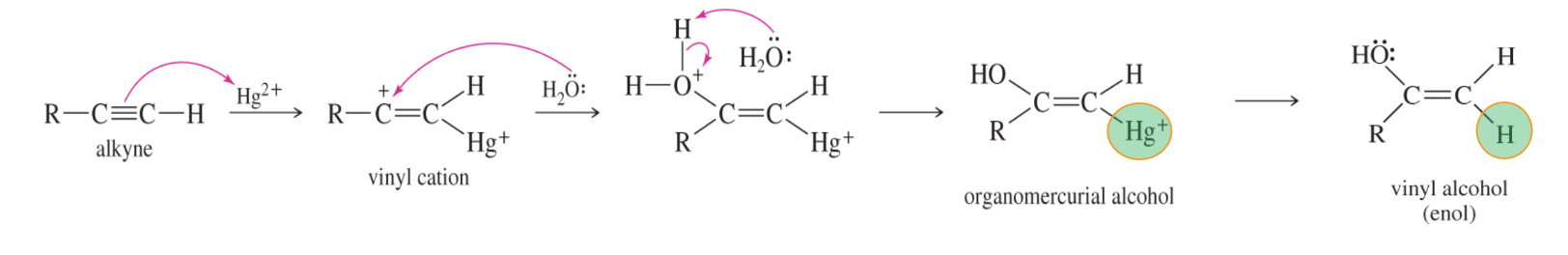
3) F. Hydration of Alkynes to Ketones and Aldehydes
Mercuric Ion-catalyzed hydrogenation
HgSO4/H2SO4
6) A. Permanganate Oxidation
KMnO4
Neutral, cold —> diketone
Terminal —> keto acids
Basic, warm —> carboxylic acids
6) B. Ozonolysis
1) O3 2) H2O
Carboxylic acids (Same as warm, basic KMnO4)
1) Formation of Alkoxide Ions
ROH + Na or K —> R-O-
2) Formation of Phenoxide Ions
PH-OH + Na or K —> PH-O-
3) Synthesis of Alcohols (Review)
A) SN reaction on alkyl halide (Ch6)
B) From Alkenes
Acid-Catalyzed Hydrogenation
Oxymercuration-demercuration
Hydroboration
Syn Dihyrdrogenation
OsO4 + H2O2
KMnO4 (cold, dilute, basic)
Anti dihydroxylation
1) mCPBA 2) H3O+
Addition of acetylide to carbonyl groups
4) Organometallic Reagents
A) Grignard
RX + Mg —> RMgX
B) Organolithium reagents
2 Li + RX —> R-Li + LiX
5) Gilman Reagents
2 RLi + CuI —> (R)2CuLi + LiX
6) Side reactions of organometallic reagents
React with acidic compounds (OH, NH, SH, C≡C-H)
React with electrophilic multiple bonds (C=O, C=N, C≡N, S=O, N=O)
7) Reduction of Carbonyl Groups
A) Sodium Borohydride (NaBH4)
Reacts with aldehydes, ketones
NOT esters or carboxylic acids
B) Lithium Aluminum hydride (LiAlH4)
Reacts with aldehydes, ketones, esters, and carboxylic acids
Much stronger
C) Rainey Ni
Adds hydrogen to ALL double bonds
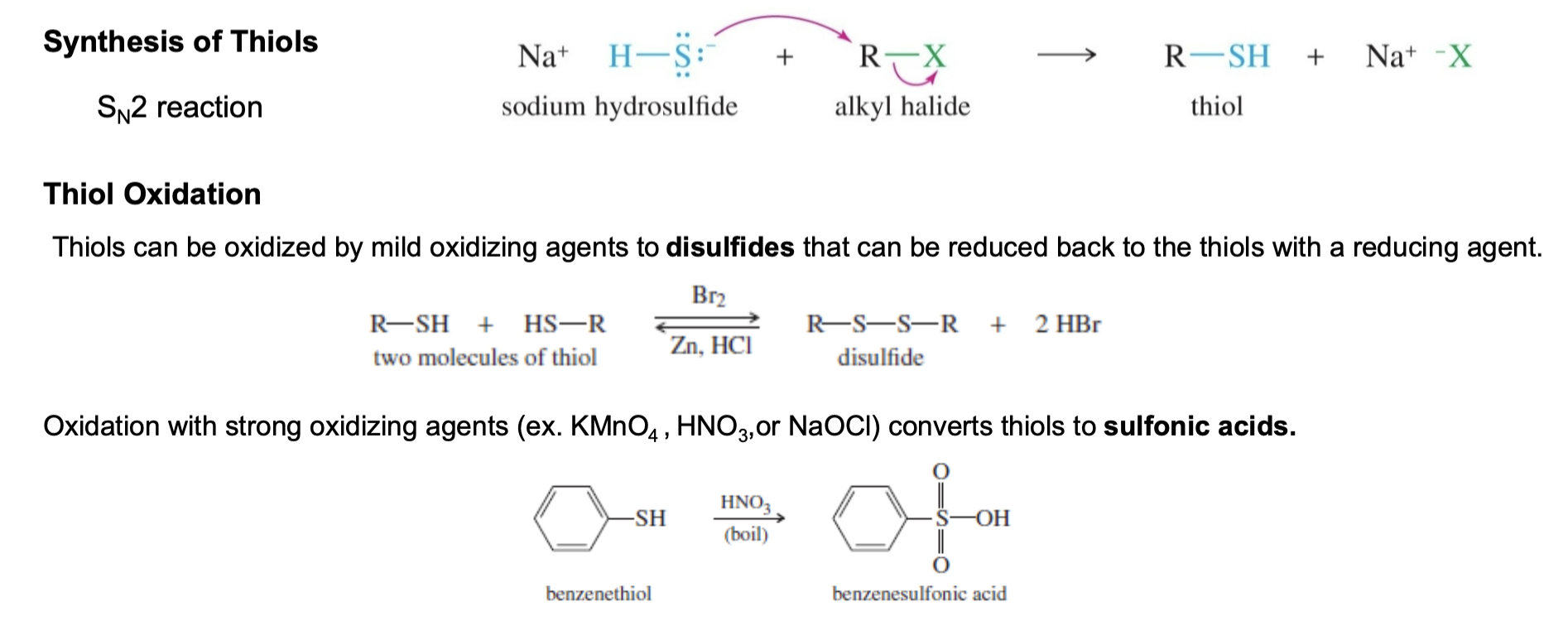
8) Thiols (mercaptans)
Synthesized though SN2 reaction with halides
Thiol oxidation:
Br2 to form disulfide
Zn, HCl to separate disulfide back into two thiol molecules
Esterification
1) Reacitons with carboxylic acids with H2SO4
2) Reactions with Acyl chloride (H of OH and Cl of acid chloride leave and bond is formed)
3) Wiliamson Ether Synthesis
Alkoxide + primary halide or tosylate
SN2