Classification of Matter
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
38 Terms
physical property
characteristic that can be observed without changing the identity of the substance

chemical property
A property that is observed when there is a change in the composition or identity of a substance

physical change
process that does not alter the composition or identity of a substance

chemical change/chemical reaction
process involving a new substance being formed

melting
The change of state from a solid to a liquid

freezing
The change of state from a liquid to a solid
evaporation
change from a liquid to a gas

condensation
process of a gas to a liquid

sublimation
process from a solid to a gas without going through the liquid state

melting point
temperature at which a solid changes to a liquid

boiling point
the temperature at which substance changes from a liquid to a gas

element
Contains one kind of atom. Simplest form of matter that cannot be broken down into simpler substances
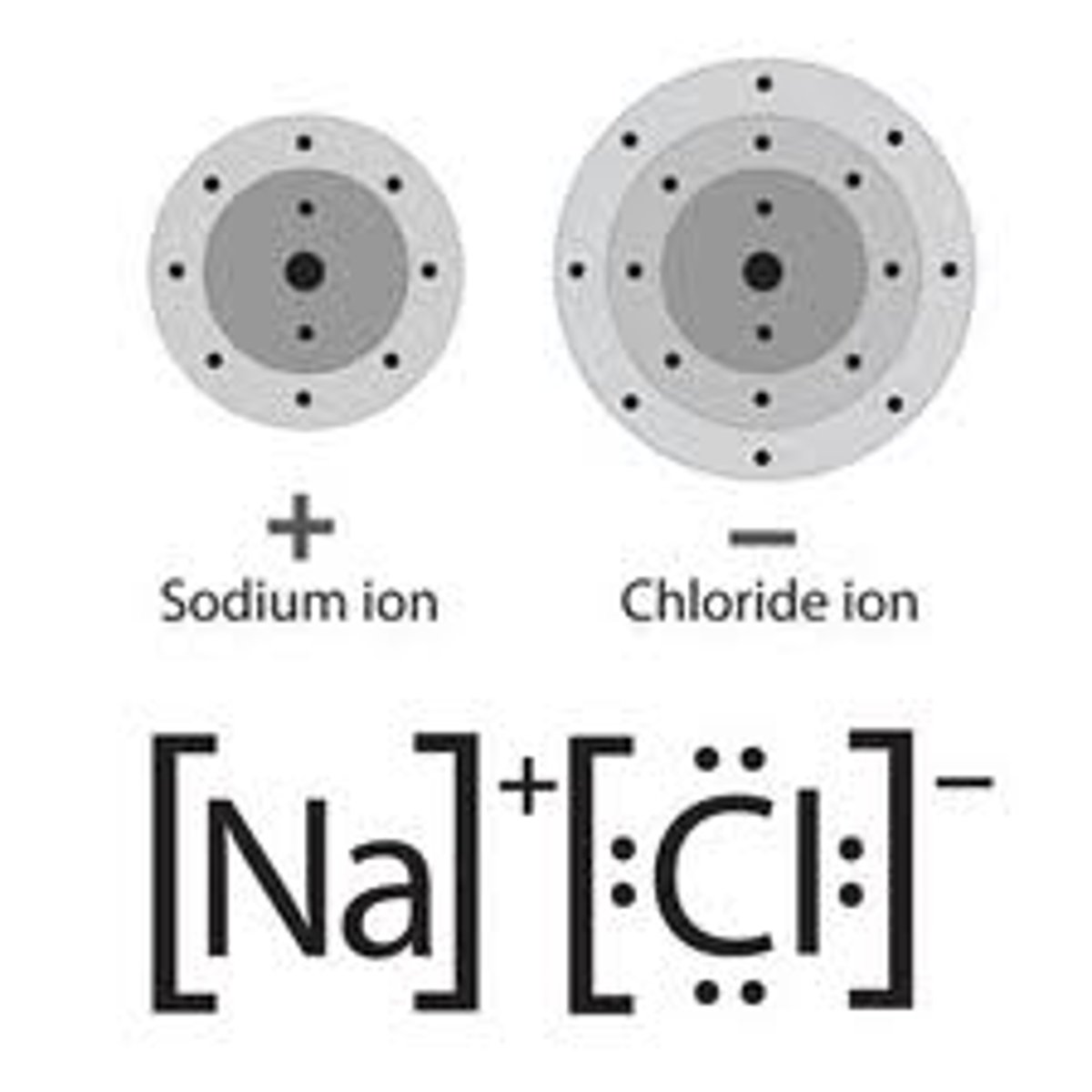
compound
Chemical combination of two or more different elements joined together in a fixed proportion.
mixture
A physical combination of two or more substances in which the basic identity of each substance is not changed

homogeneous mixture
Mixtures with a uniform composition. Also known as a solution.

heterogeneous mixture
a mixture that does not have a uniform composition.

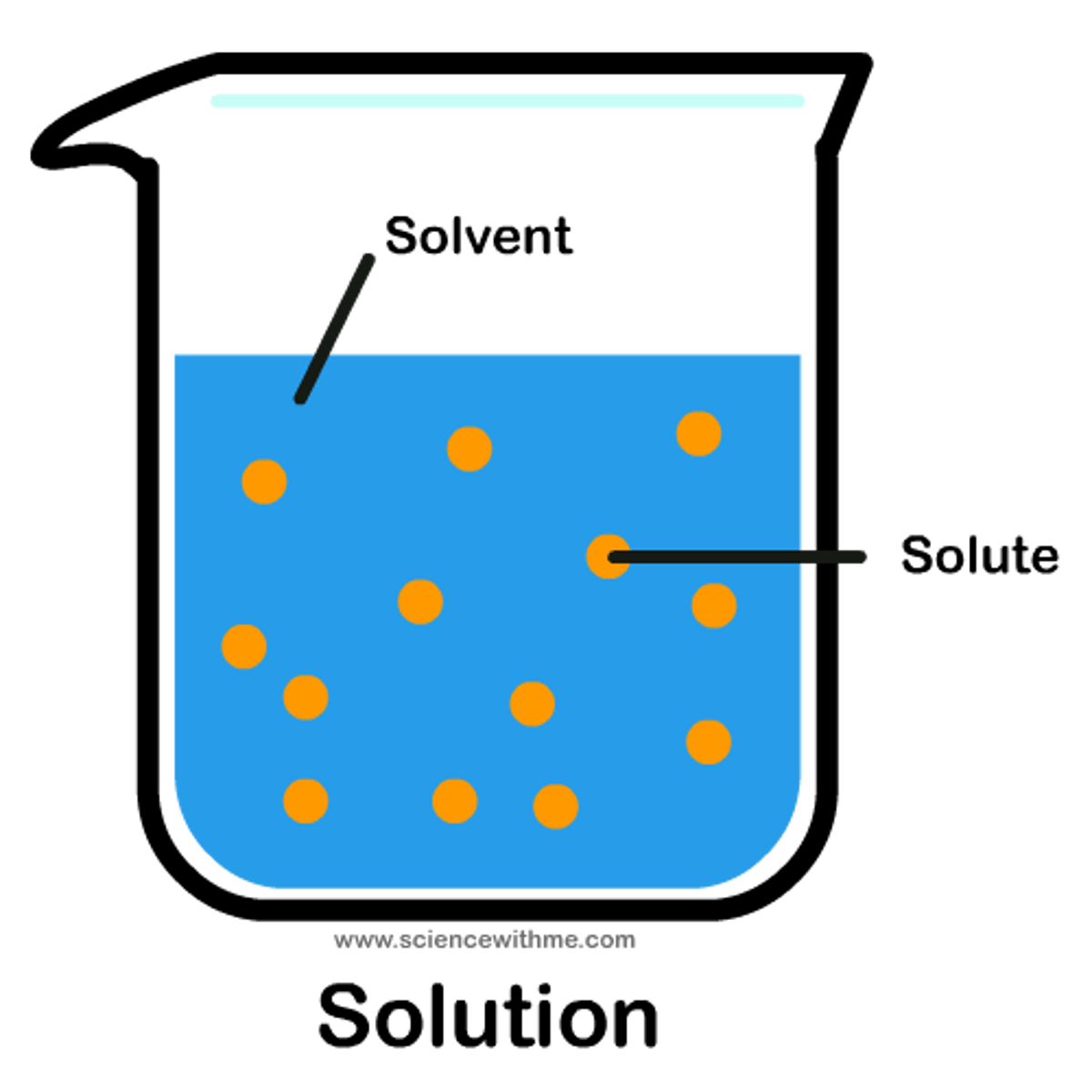
solution
Another word for homogeneous mixture. Alloys are a solid example of this.
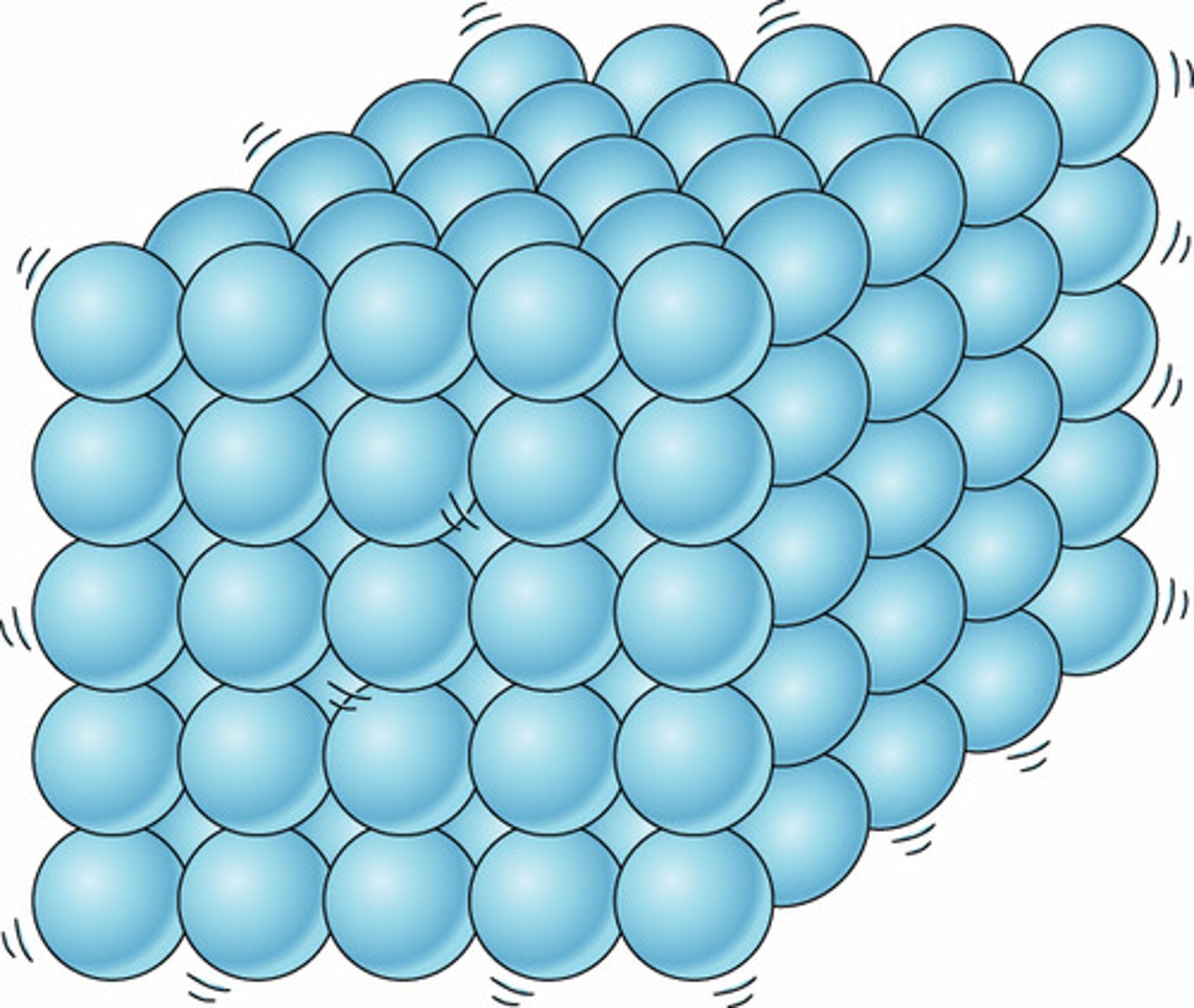
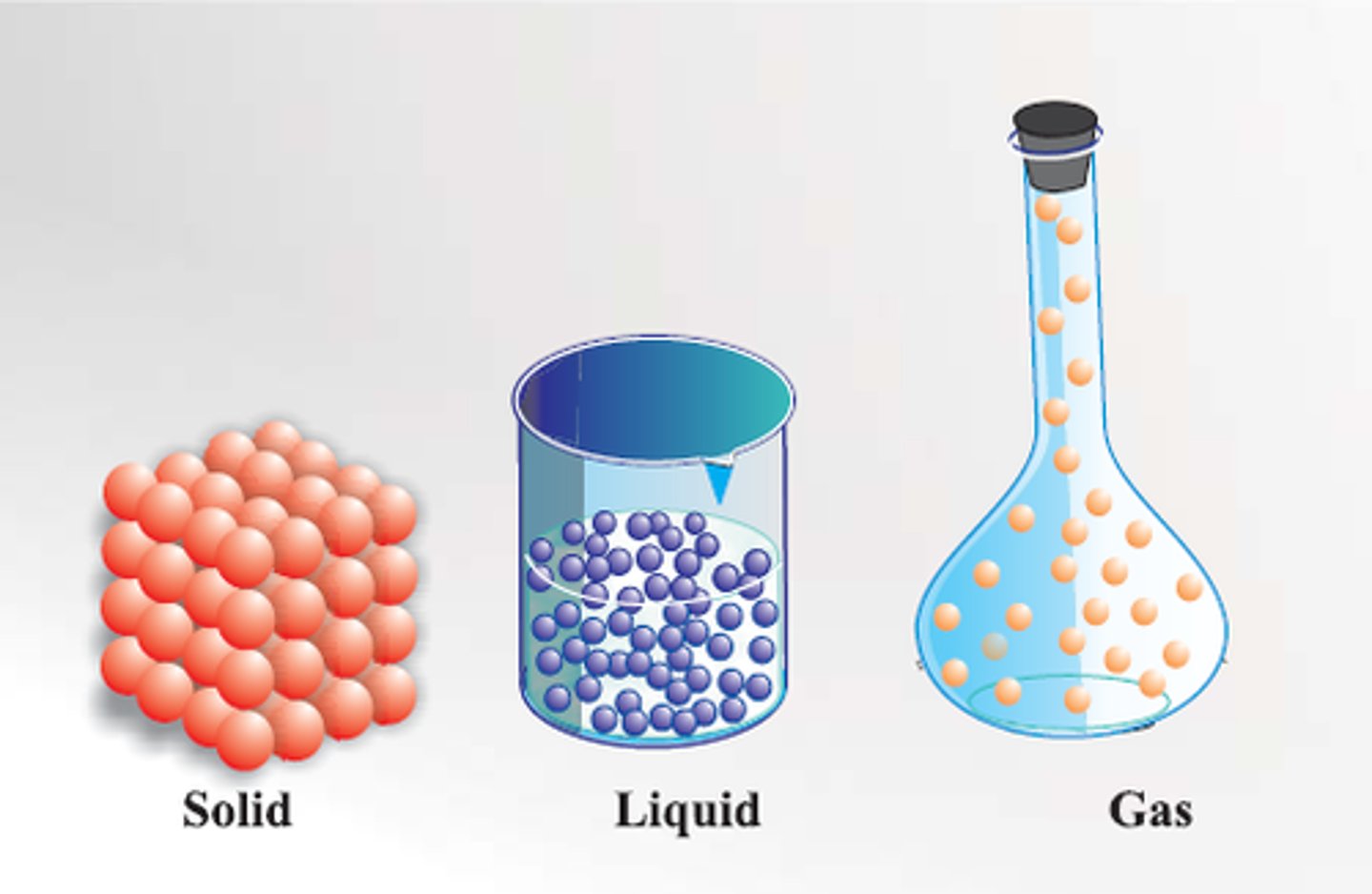
solid
definite shape and volume; close together and little movement
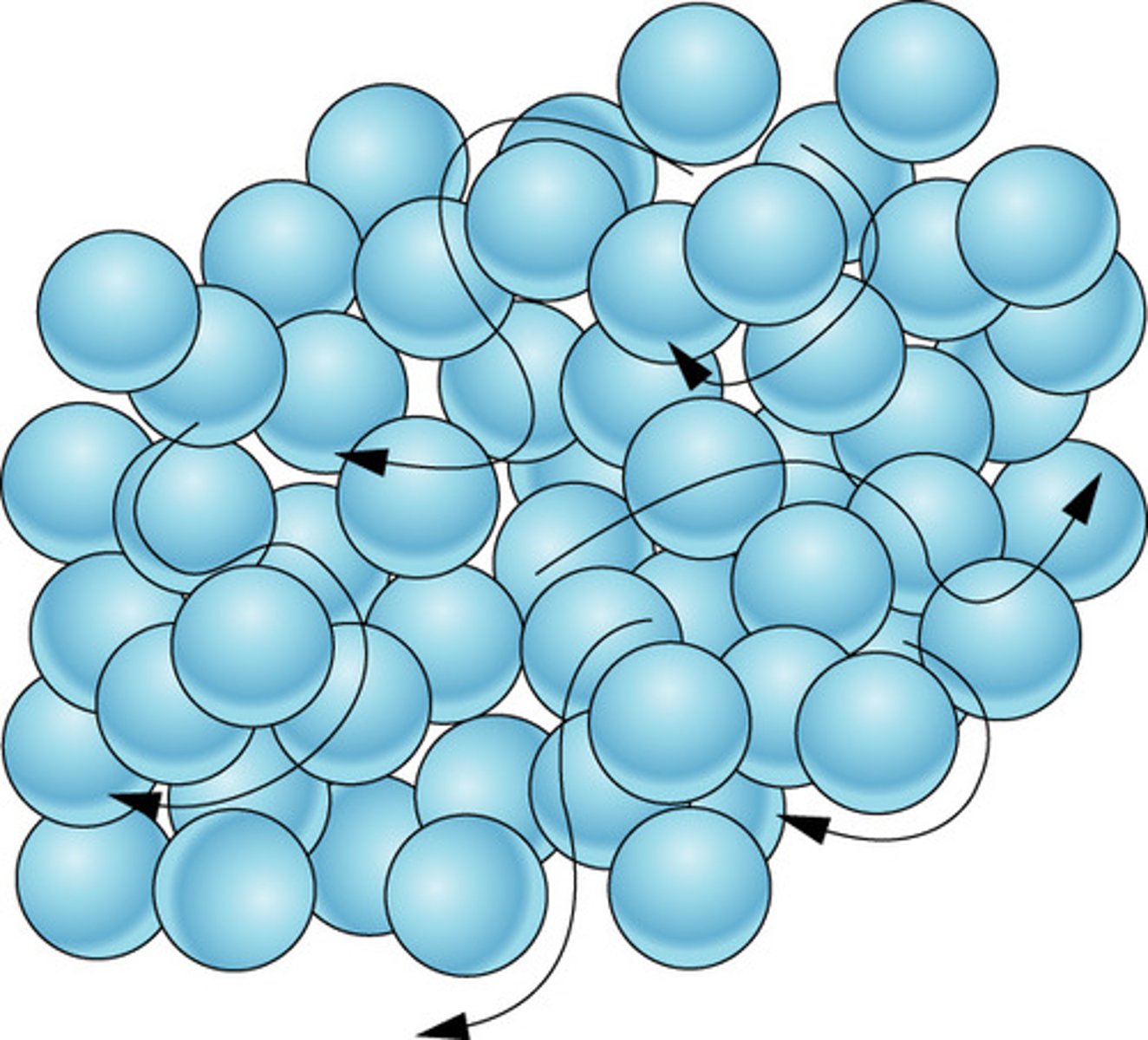
liquid
definite volume but no definite shape; some movement
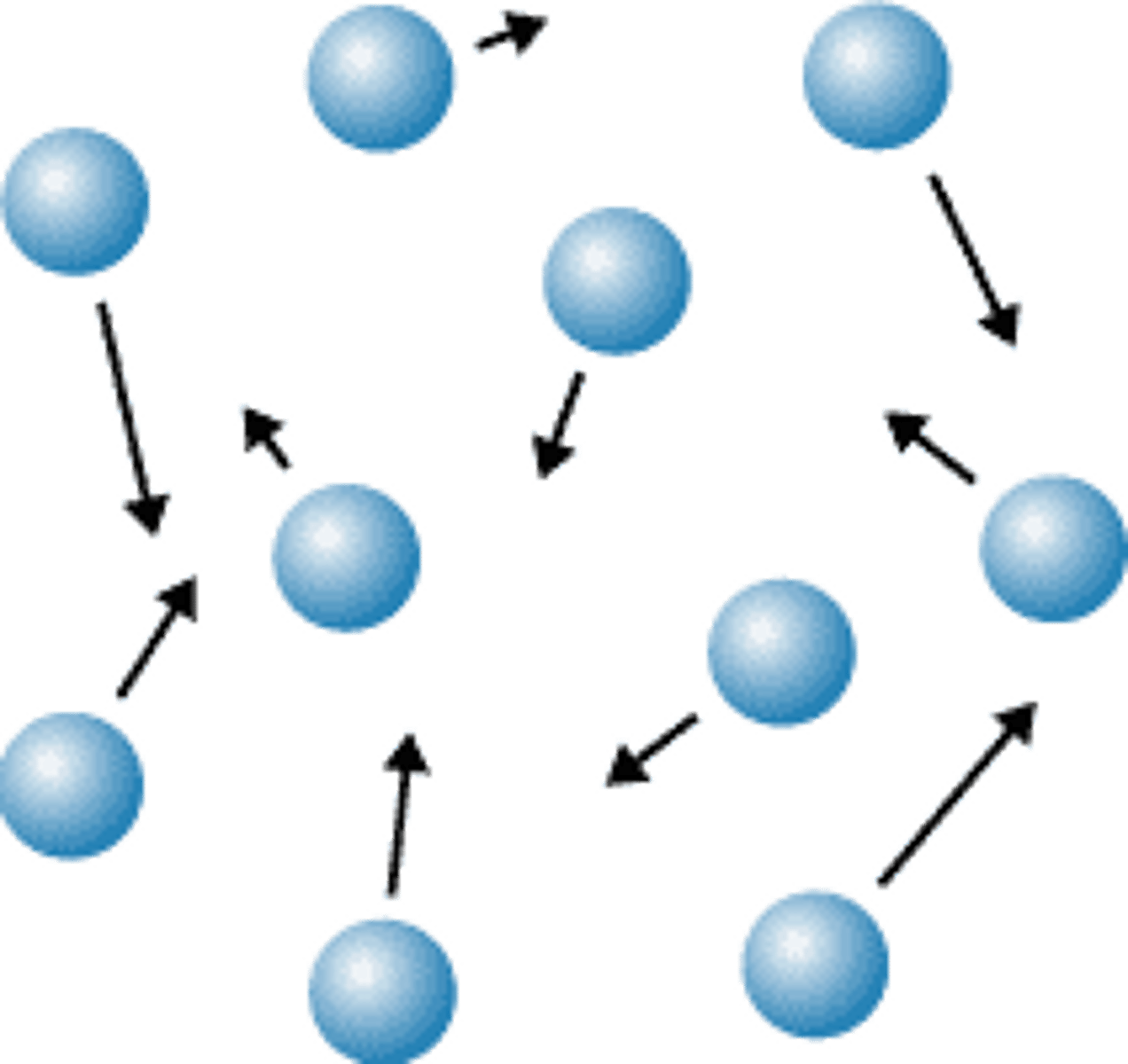
gas
no definite volume or shape; particles move fast and are far apart
deposition
process from a gas to a solid without going through the liquid state

matter
Anything that has mass and takes up space
atom
Smallest particle of matter that retain their own unique properties. Smallest particle of an element

molecule
A chemical combination of two or more atoms to create the smallest particle of a given compound
Chemistry
The science that investigates and explains the structure and properties of matter
Mass
The amount of matter that an object contains
Properties
characteristics that describe matter
Pure Substance
matter with the same fixed composition and properties. Could be an element or a compound.
Chemical formula
Combination of chemical symbols that show what elements make up a compound and how many of each type of atom are present
solvent
substance that dissolves the solute
solute
substance that is dissolved in a solution
Alloys
a solid solution that contains metallic elements
Chemical symbol
one, two, or three letter abbreviation for an element
Bond
a chemical connection between two or more atoms
Fermenting, rusting, oxidation, and burning are examples of this
Chemical change
Oxidation state, flammability, and toxicity are examples of this
Chemical property
Phase changes, changes in size, and changes in shape are examples of this
Physical change
Color, malleability, ductility, and density are examples of this
Physical properties