hnsc 1210 unit 8 - water and minerals
1/133
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
134 Terms
water
most indispensable of all nutrients
need more water everyday than any other nutrients; 50 times more water than protein and 5,000 times more water than Vitamin C
can only survive a few days without water
where can you find water in the body
veins
arteries
capillaries
incorporated into chemical structures of compounds that form the cells, tissues, and organs of the body
water participated readily in many chemical reactions
water weight
makes up 60% of adults body weight, 90lbs in a 150lbs in a person
amounts of water in body can vary overtime
eating argue sodium contents can increase temporarily the body’s water content
typically water shed the excess over a day or so as the sodium is excreted
does not reflect a change in body fat, takes days or weeks
water weight can change over night
functions of water
transport vehicle for nutrients and wastes
universal solvent
body cleansing agent
lubricant/cushion for joints and protection for sensitive tissue
maintenance of body temperature
functions of water: transport vehicle for nutrients and waste
Water brings requirements to cells and carries away the end products from cells. Without water, cells quickly die.
functions of water: universal solvent
Water dissolves amino acids, glucose, minerals and many other substances needed by cells.
function of water: body’s cleansing agent
Wastes such as nitrogen dissolve in the watery blood, and the kidneys filter these wastes from the blood and excrete them, mixed with water, as urine.
functions of water: lubricant/cushion for joints and protection for sensitive tissue
This is because water molecules resist being crowded together – they spread out. It can protect sensitive tissue like the spinal cord from shock (e.g., amniotic fluid protects the fetus from shock). Water also lubricates all tissues that are moistened with mucus (e.g., digestive tract, respiratory tract lining).
functions of water: maintenance of body temperature
Sweat is the body’s coolant. To rid the body of excess heat, blood is routed through the capillaries just under the skin, the skin secretes sweat, and this water evaporates. Converting water to vapor takes energy; therefore heat energy decreases, cooling the skin and the underlying blood. The cooled blood then travels to the body’s core.
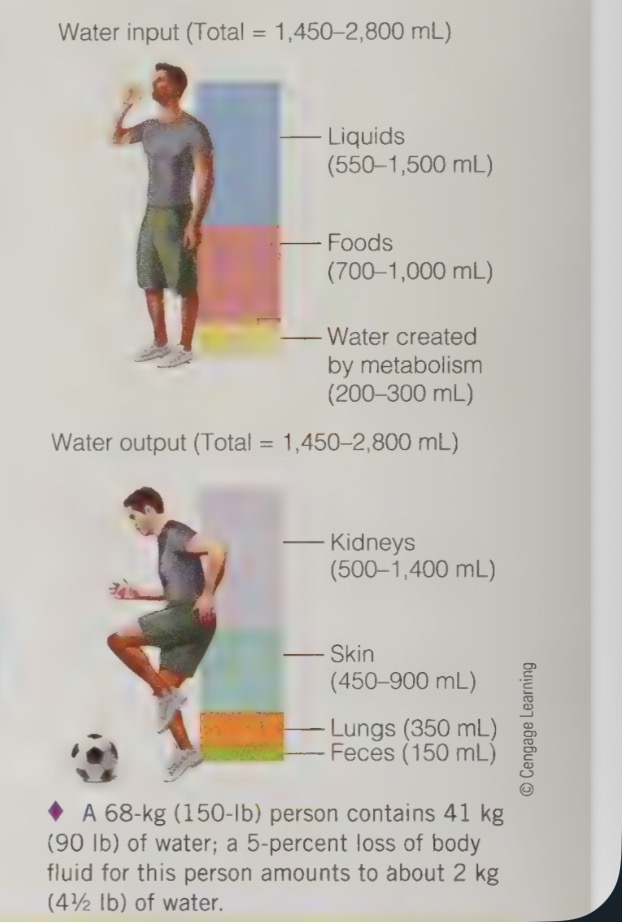
water balance
the body loses water everyday through urine, sweat, respiration, and our feces
therefore person must consume at least the same amount that is lost, to maintain water balance, and avoid complications
water balance - a typical example
each day water enters the body in liquids and foods, and some water is created in body as a by-product of metabolic processes
water leaves the body through the evaporation of sweat, in the moisture of exhaled breath, in the urine, and in the feces
what regulates water intake
thirst and satiety
when the blood becomes too concentrated because water has been lost, the molecules/particles in the blood start to attract water from the salivary glands, causing the mouth to dry
how does the hypothalamus monitor blood concentrations ?
signals the pituitary gland to release a hormone that causes he kidneys to shift water back into the blood stream, rather than package the water for excretion as urine
if blood is too concerted or i flood volume or blood pressure drop too low, the hypothalamus initiates nerve impulses to the brain that register as thirst
thirst is the first sign of dehydration but by the time you feel thirsty the body has already lost up to two cups of fluid and the need for fluids is urgent
in older adults, the perception of thirst is lost, so they should actively try to consume fluids throughout their day regardless of thirst
dehydration
occurs when there is loss of body water and the water is not replaced
first sign is thirst, the body is dehydrated
a loss of 5% of our body fluids can trigger symptoms such as headache, fatigue, confusion, and forgetfulness. it can even cause an elevation in heart rate
body starts to conserve fluids so less urine is excreted and sweet in ceases
fluid is diverted into the blood vessels to support blood pressure levels
sweating has ceased, body heat starts to build up
when we lose more than 5% of our body fluids, we risk serious complications such as shock, seizures, coma, and even death
chronic low fluid intake
increase our risk for developing bladder, colon, and other cancers, heart problems, gallstones, kidney stones, and urinary tract infections

water intoxication
occurs when too much plain water in consumed and floods the body fluids, disturbing he normal concentration
incipient is rare, but can cause symptoms such as headache, muscular weakness, lack of concentration, poor memory, and appetite loss
in severe cases, death
how much water do we need?
amount of water our body depends on a number of factors, such as foods eaten, activity level, temperature of environment etc.
DRI recommendations for fluid are general guideline and assume normal diet and moderate temps.
80% of our daily need for fluids
remainder comes from food we eat
most foods contain at least some water
body makes small % of day’s fluid, as energy break down they release some water as byproduct
fluid intake caffeinated beverages
do count towards fluid intake
even though they are diuretics, the actual net loss of fluid fro the body is very small
some people also adapt diuretic effects, loosing no additional fluids
other fluids include, soft drinks, juice, jello, pudding, soup, popsicles, ice cream, milk etc.
increased fluid needs
sweating and physical activity can increase the need for fluid
factors that increases fluid needs

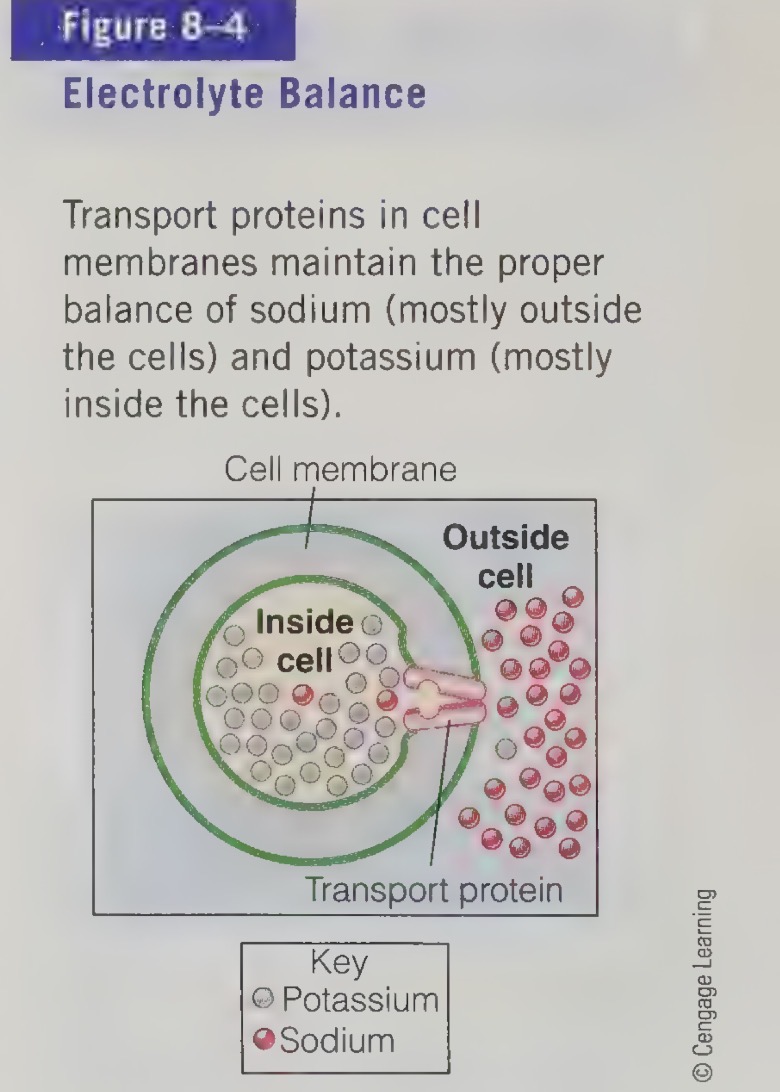
body fluids and minerals
most body’s water is contained inside the cells
some water on outside of the cells and the remainder fills up the blood vessels
water slips across cell membrane freely, so the cells cannot regulate the amount of water directly
pump minerals across their membrane
major minerals form salad (compounds composed of charged particles)
dissolve in body fluids
the cells direct where the salt go and this determines where the fluid flows because water follows salt
ensures cells do not collapse when water leaves, or swell and burst when water enters the cells direct where
salt dissolve
when salt dissolve in water they separate into single electrical charged particles called ions
dissolved in water ions carry electrical current because of this are called electrolytes
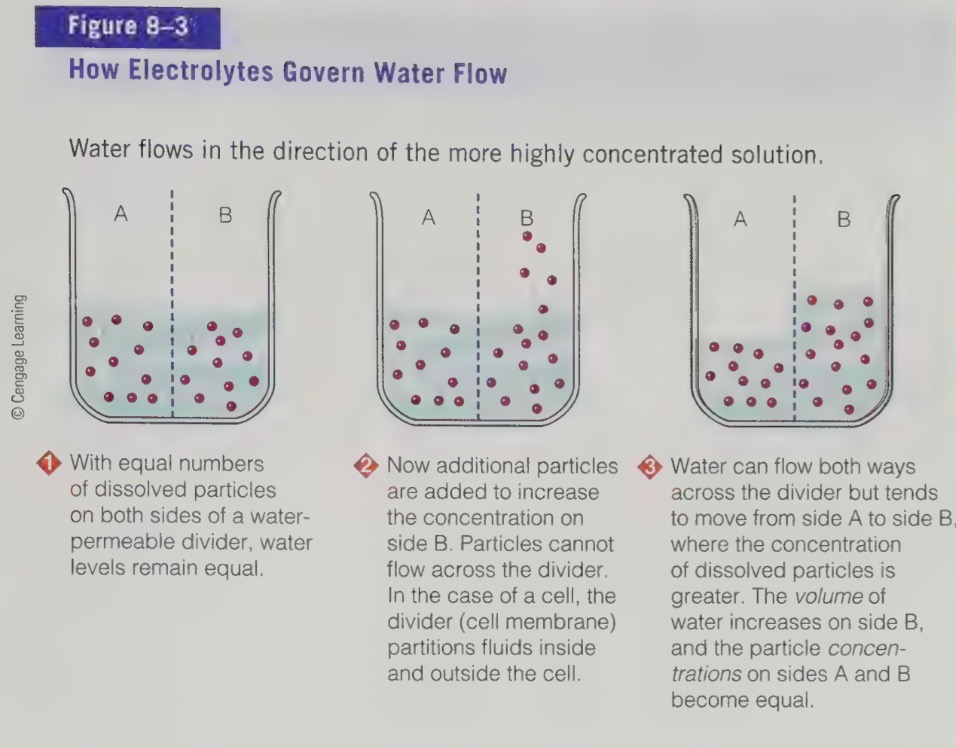
electrolytes
Compounds that partially dissociate in water to form ions (electrically charged particles)
dissolved electrolytes present in unequal concentrations
on either side of a water permeable membrane, the water flows onwards the more concentrated side to equal concentrations
process also occurs in body
to control flow of water, body must use energy to move the electrolytes from one compartment to another
transport of proteins in cell membrane that form pumps to move mineral ions across the cell membranes
as a result we have fluid and electrolyte balance occurs
fluid and electrolyte balance
Maintenance of the appropriate kinds and amounts of fluids and mineral in each compartment of the body.
if fluid balance is disturbed
serve illness can develop quitclaim because fluid will shift from one compartment to another
ex. committing and diarhhhea the body is losing water form the digestive tract, water is pulled from between cells in every part of the body
kidney detect water loss and try to retrieve water that is destined for excretion by raising sodium concentration, causing more water to be pulled our of them
fluid and electrolyte imbalance occurs as well, medical emergency
the loss of fluid in cell disruptions the heart beat and threatens life
one cause of death as a result of ED
minerals
Naturally occurring, inorganic, essential, chemical elements.
major minerals are found in the human body in amounts larger than
5 grams
seven major minerals
calcium (Ca)
phosphorus (P)
magnesium (Mg)
sodium (Na)
potassium (K)
chloride (Cl)
sulphate (S)
trace mineral are found in the human body in amounts less than
5 grams
nine trace minerals
iodine (I)
iron (Fe)
zinc (Zn)
copper (Cu)
selenium (Se)
fluoride (F)
chromium (Cr)
manganese (Mn)
molybdenum (Mo)
minerals general functions
Electrolytes: Na, K, Cl
maintain water balance in cells & blood
Na & K: used in muscle contractions &. nerve transmission
Body structure: Ca, P, Mg, F, Zn
bones, teeth
Structure of DNA, RNA, phospholipids and ATP (energy molecule): P
Protein structure: Fe, Zn, Ca, Se, I
the protein and the element are bound to make a specific shape and thus function, e.g.,
Fe & hemoglobin, I & thyroid hormone
Cell signaling & communication: Ca, Na
Calcium moves across cells as a messenger, stimulating proteins and cell activity
Antioxidant defense: Zn, Cu, Mn, Se, S
components of defense enzymes
calcium
DRI = RDA: 1,000 mg/d
DRI recommendations for children and adolescents are higher to achieve peak bone mass
peak bone mass
The highest attainable bone density a person can achieve. Usually reached by the late twenties, early thirties.
calcium functions
most abundant mineral in our body
almost all is stored in bones and teeth
1% of body’s calcium is in the fluids inside and outside of cells
tiny amounts of calcium play some major roles
Regulates the movement of ions across cell membranes and is also important for nerve transmission.
Helps to maintain normal blood pressure levels: an increase in dietary calcium correlates with a decrease in blood pressure in both healthy individuals and those with hypertension.
Is essential for muscle contraction and therefore for heartbeat.
Plays an essential role in blood clotting.
Allows for the secretion of hormones, neurotransmitters, and digestive enzymes.
Activates cellular enzymes that regulate many processes in the body.
because of important functions, blood calcium is
tightly regulated
if diet is not adequate in calcium, body will maintain blood calcium levels by taking calcium from bones
calcium in bones and teeth
plays 2 important roles
integral part of bone structure, calcium and phosphorus are essential for bone formation, form hydroxide-appetite crystals that invade collagen to lend more rigid to children developing bones. teeth formed a similar way: hydroxide crystals form on a collagen matrix that create the dentin that gives strength to the teeth. the turnover of minerals in the teeth is not as rapid as in the bone, but does carry out through life
serves as a bank that can release calcium to the body if blood calcium level drops, calcium moves in and out of the bone every minute of everyday
calcium food sources
milk
leafy green vegetables (broccoli, kale, rutabaga, beet greens, turnip greens, bok choy, brussels sprouts, cauliflower, cabbage, watercress) - fairly absorbed
canned sardines
canned salmon (bones contain calcium and are softened in canning process)
almost
ca-se tofu
ca-diet fortified juices
fortified beverages
fortified rice or nut based beverages
calcium regulation
when increased need for calcium, body adjusts by increasing calcium absorption in the intestine, prevent loss from the kidneys and may mobilize calcium from the bone
infants, children, and pregnant women all ave higher needs for calcium so the body absorbed more calcium consumed
how much do children approx consume of calcium
60% of the calcium they consume
approx how much calcium do pregnant women
50%
approx healthy adults more calcium consumed
25%
adult defilement in calcium for months
may double their calcium absorption
if same person is supplied with calcium for years, so their calcium absorption decrease and may only absorbed 1/3 of normal amount
adjustment take time - suddenly cutting back on calcium intake will most likely lead to bone calcium loss
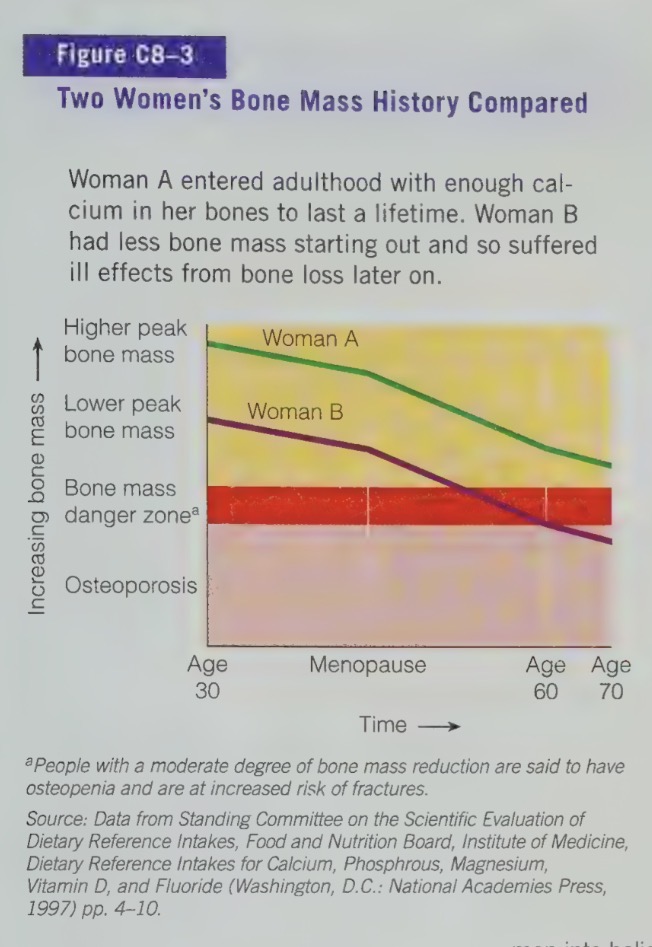
bone loss is inevitable as we age
bone loss can be slowed by a diet high in calcium, alongside with sufficient physical activity
to protect against bone loss
high calcium intakes are recommended early in life
by about twenties - early thirties, the skeleton has reached peak bone mass and is no longer adding significant bone density.
if a person has a calcium-poor diet during their growing years, their peak bone mass will not be maximized
a person with a lower peak bone mass is more likely to develop fragile bones, adult bone loss and osteoporosis
calcium deficiency
osteoporosis is characterized by weak, brittle bones which can result in fractures
calcium toxicity
UL: 2,500 mg/d
symptoms of calcium toxicity include constipation, interference, with the absorption of other minerals, and an increased risk of kidney stone formation
phosphorus recommendations
second most abundant mineral in the body
DRI: RDA: 700 mg/d
phosphorus functions
P salts are critical buffers, helping to maintain the acid-base balance of cellular
P is part of every cells DNA and RNA. Therefore it is essential for growth and renewal of tissues.
P compounds carry, store and release energy during the metabolism of energy containing nutrients.
P compounds help many enzymes and vitamins to extract energy from CHO, protein and fat.
P forms part of the molecules that are part of phospholipids.
P is also present in some proteins.
approx how much of the body’s phosphorus is stored in the bones and teeth
85%
packaged with calcium into hydroxyapatite
phosphorus food sources
milk products are a source of phosphorus is stored in the bones and teeth, packaged with calcium into hydroxyapatite crystals
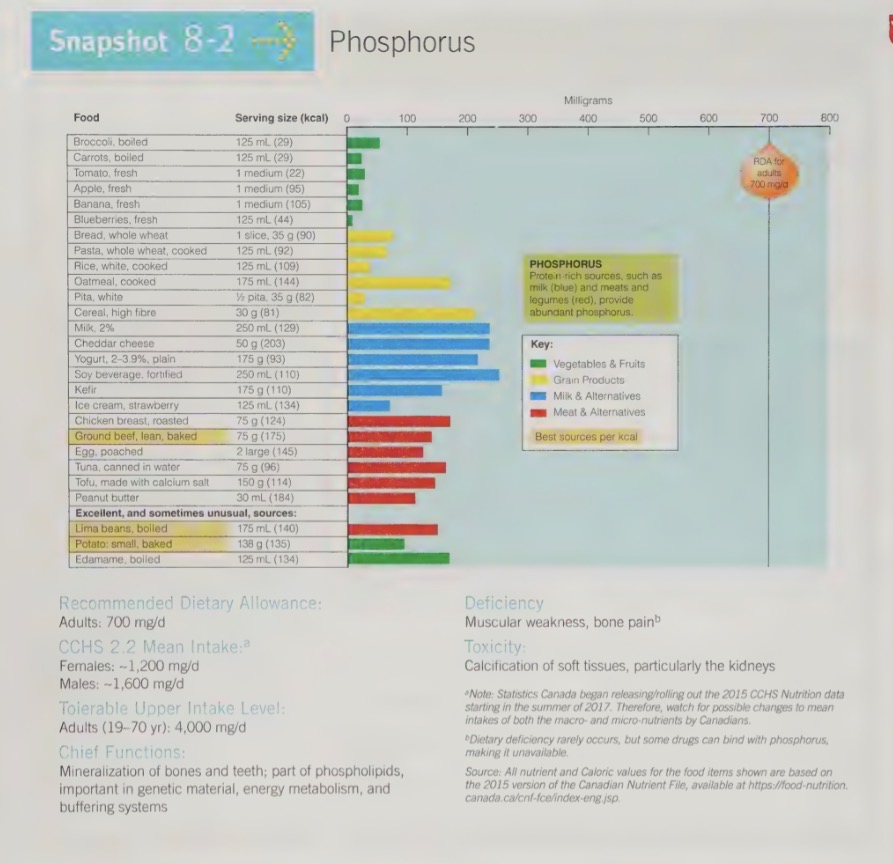
phosphorus toxicity
UL: 4,000 mg/d
toxicity can cause calcification of non-skeletal tissues (especially in kidneys)
magnesium recommendations
DRI = RDA: men - 400mg/day, women - 310mg/day
magnesium functions
critical to many cell functions:
Assists in the operation of more than 300 enzymes.
Is needed for the release and use of energy from CHO, protein and fat.
Directly affects the metabolism of other nutrients, including phosphorus, calcium and vitamin D.
Acts in the cells of all soft tissues, forming part of the protein making machinery.
Works with calcium in proper functioning of muscles – magnesium helps muscles relax after calcium causes contraction.
In the teeth, magnesium holds calcium in tooth enamel, promoting resistance to tooth decay.
over half of the magnesium in the body is stored in the
bones
when blood levels of magnesium are low, the body can borrow from the bone stores of magnesium
kidneys work to conserve magnesium
magnesium food sources
nut
legumes
seafood
whole grains
dark green vegetables
chocolate
are all significant sources of magnesium
Mg is easily washed and peeled away from food during processing; therefore whole foods are better source
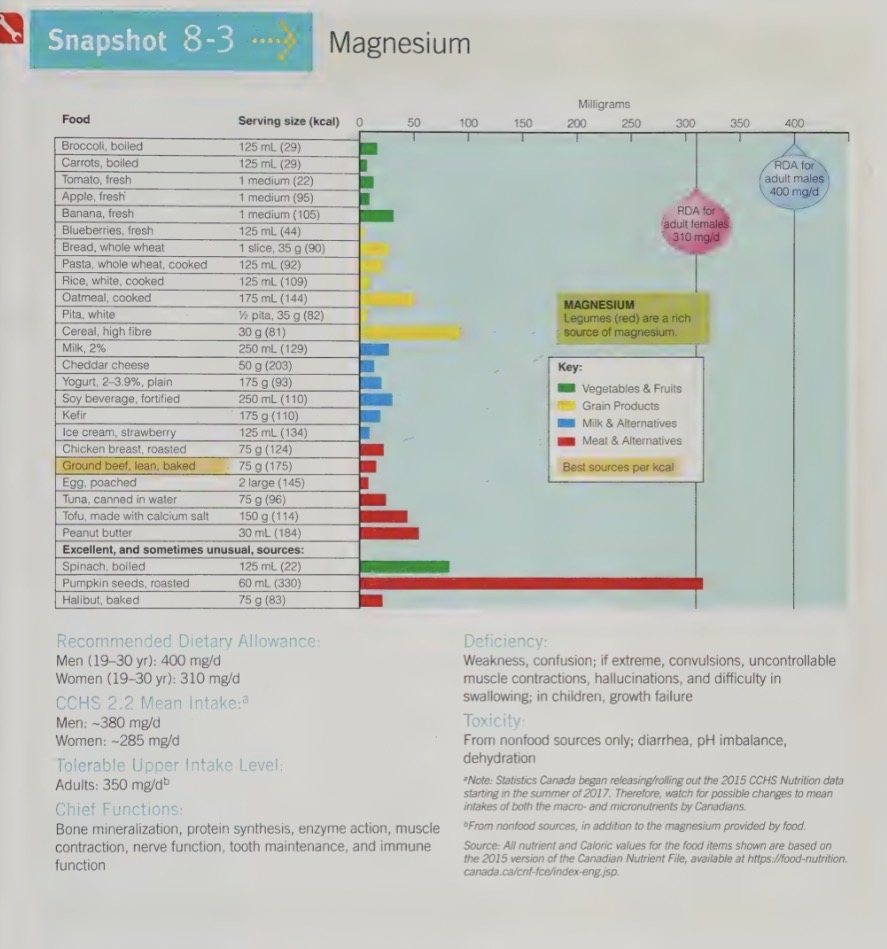
magnesium deficiency
can result of inadequate intake, but also result from committing, diarrhea, alcoholism or protein malnutrition
symptoms although rare, include weakness and confusion
in extreme cases, uncontrollable muscle contractions (heart unable to stop spasms once it starts), hallucinations (sometimes mistaken for mental illness or drunkenness), swallowing difficulties, and growth failure
evidence shows that even lower Mg intake (below DRI, but no causing true deficiency) may increase the risk for disease development (e.g populations studies have shown a lower incidence of death from heart failure in area where drinking water provides Mg)
magnesium toxicity
UL: 350mg/day (supplement/ drugs only; not food magnesium)
toxicity is rare but can be fetal
is a result of of non-food sources of Mg for ex. supplements of Mg salts
accidental poisoning sometimes occurs when children get into medicine cabinets and ingest large doses of Mg contain educations, such as laxatives and antacids
older adult who abuse these medications are also at risk of toxicity
symptoms include severe diarrhea, dehydration, and acid-base imbalances
sodium recommendations
DRI recommendations for Na are 1,500mg for adults and the UL is 2,300mg/d
may seem like a lot but the average canadian diet contains over 3,100mg of sodium

sodium (Na) is a positively harmed ion that is used to maintain the volume of fluid outside of cells. other functions include
maintaining acid-base balance;
is essential for muscle contractions; and
is essential for nerve transmission.
sodium and body fluids
when a person eats a salted food, thirst ensures that the person will drink water until the sodium to water ratio n the body is restored
kidney excrete the excess Na along with water
if blood Na drops, body water is lost, and both water and sodium must be replenished to avert an emergency
overly strict use of low-sodium diets to treat hypertension, kidney disease or heart disease can deplete the body of needed Na, as can committing, diarrhea, and extreme heavy sweating
scientists believe that about 30-40% of the Na in our body is stored on the surface of bone crystal, so that it is easy for the body to draw on if blood Na concentrations need to be replenished
sodium food sources
part of table salt (NaCl) and makes up 40% of its weight (1g salt contains 400mg)
greatest contributors of sodium in our diet are processed and fast foods
about 77% of our sodium intake comes from processed and fast foods
salt added during cooking and at the table contributes 11% of sodium intake, while 12% occurs naturally in foods
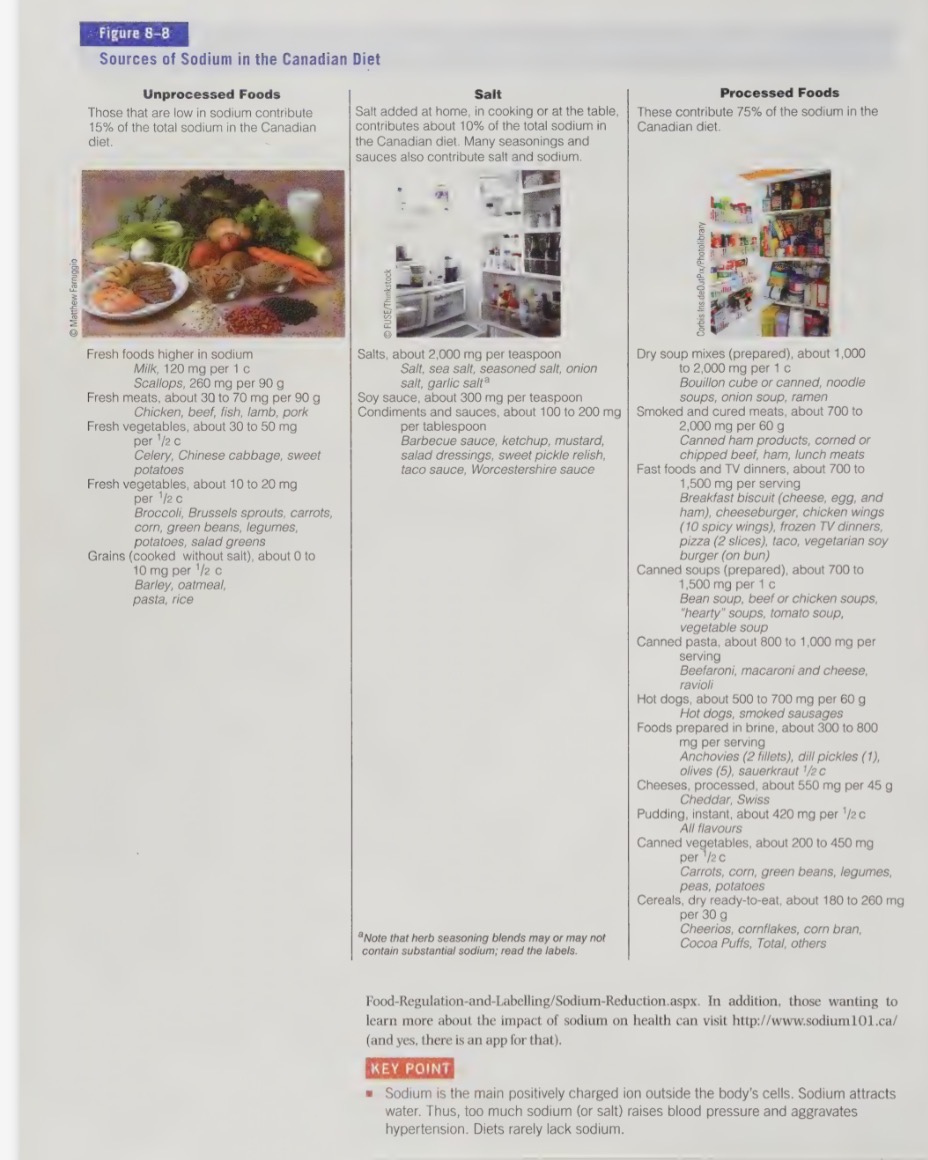
sodium deficiency
harmful to our health, however no known human diets lacks Na
body absorbs Na freely and most food contain more Na than needed
kidney can conserve some Na to return the bloodstream in the event of rare deficiency
symptoms include muscle cramps, mental apathy, and loss of appetite
sodium toxicity
can lead to hypertension (high blood pressure)
world-wide, populations with high salt intakes experience higher rates of hypertension (HTN), CVD, and cerebral hemorrhage (a form of stroke that is hypertension related)
there is a direct relationship between sodium and blood pressure
as sodium intakes increase, the average blood pressure levels also increase, and as blood pressure rises, so does the risk of death from cardiovascular disease (CVD)
even small increases in Na intake have this effect
the effect of Na on blood pressure is seen more strongly in salt-sensitive individuals…
including those with diabetes, hypertension or kidney disease, those of African-American decent, those with a family history of high blood pressure and anyone over the age of 50,
because blood pressure responds more dramatically to salt in older age.
one proven dietary approach to help people lower there Na intake and increase their potassium intake is called DASH DIET
(dietary approaches to stop hypertension)
emphasis on vegetables and fruits, with adequate amounts of nuts, fish, whole grains, and low-fat dairy products
red meats, butts, high-fat foods and sweets are de-emphasized
salt and sodium are greatly reduced in this diet plan
with emphasis on veggies and fruit, potassium levels are increased
low potassium intake on its own raises blood pressure, whereas high potassium intake can prevent or even correct hypertension
regular physical activity can also lower blood pressure
excess sodium can cause other problems as well
It can increase the amount of calcium excreted.
May directly stress a weakened heart or aggravate existing kidney problems (this is why sodium restriction is often part of the treatment for heart disease or kidney disease).
Researchers are looking at whether high salt intake is the reason for high rates of stomach cancer in those of Asian decent (many sauces and flavorings used in Asian cooking (e.g., soy sauce, MSG) are very high in Na
reducing sodium intake
cutting back on Na intake can b easier then people think
replacing a few foods consumed can have big impact on daily Na intake
in just omitting the sauce from a sandwich, we can save 700mg of Na

many benefits of reducing dietary Na
ex. many older adults without hypertension die of stroke
reducing dietary Na may lower blood pressure enough to reduce the risk of strokes
food may seem less tasty at first
takes our bodies some time for our tastes to adjust (at least 3 weeks), but soon natural flavour of the food will become the preferred taste
important to cut back on processed, conscience, and fast food (source of majority of salt in the diet)
important to read the nutrition facts panels on foods - even foods that dont taste salty contain sodium (eg. packaged puddings, many breakfast cereals, etc
unprocessed foods are not only lower in sodium, they’re typically higher in potassium; both which can benefit blood pressure
potassium recommendations
DRI recommendation is 4,700 mg/d
if following a healthy balanced diet we shouldn’t have a robles meeting the needs
veggie and fruit intake in low in CAD and USD
typically US diet only contains 1/2 of DRI recommendations
blood levels remain normal even with reduce dietary intake, however risk for disease do increase
potassium functions
Is the major positively charged ion found inside all living cells.
Plays a critical role in the maintenance of fluid and electrolyte balance in the body.
Plays a critical role in the maintenance of cell integrity.
Is critical for the maintenance of the heart beat through its role in the contraction of muscles. Sudden deaths during fasts, as a result of eating disorders, after prolonged, severe diarrhea or with kwashiorkor are often the result of heart failure caused by potassium loss
dehydration causes a loss of K from inside cells
this condition is dangerously, because once the cells of the brain lose K, they lose the ability to detect the need for fluids
this is why people should only take diuretics under doctor will encourage the patient to eat potassium rich foods to compensate for the losses
potassium food sources
richest sources are fresh, whole foods, as processing causes potassium loss
most fruits and vegetables are great source of potassium and legumes are also a source
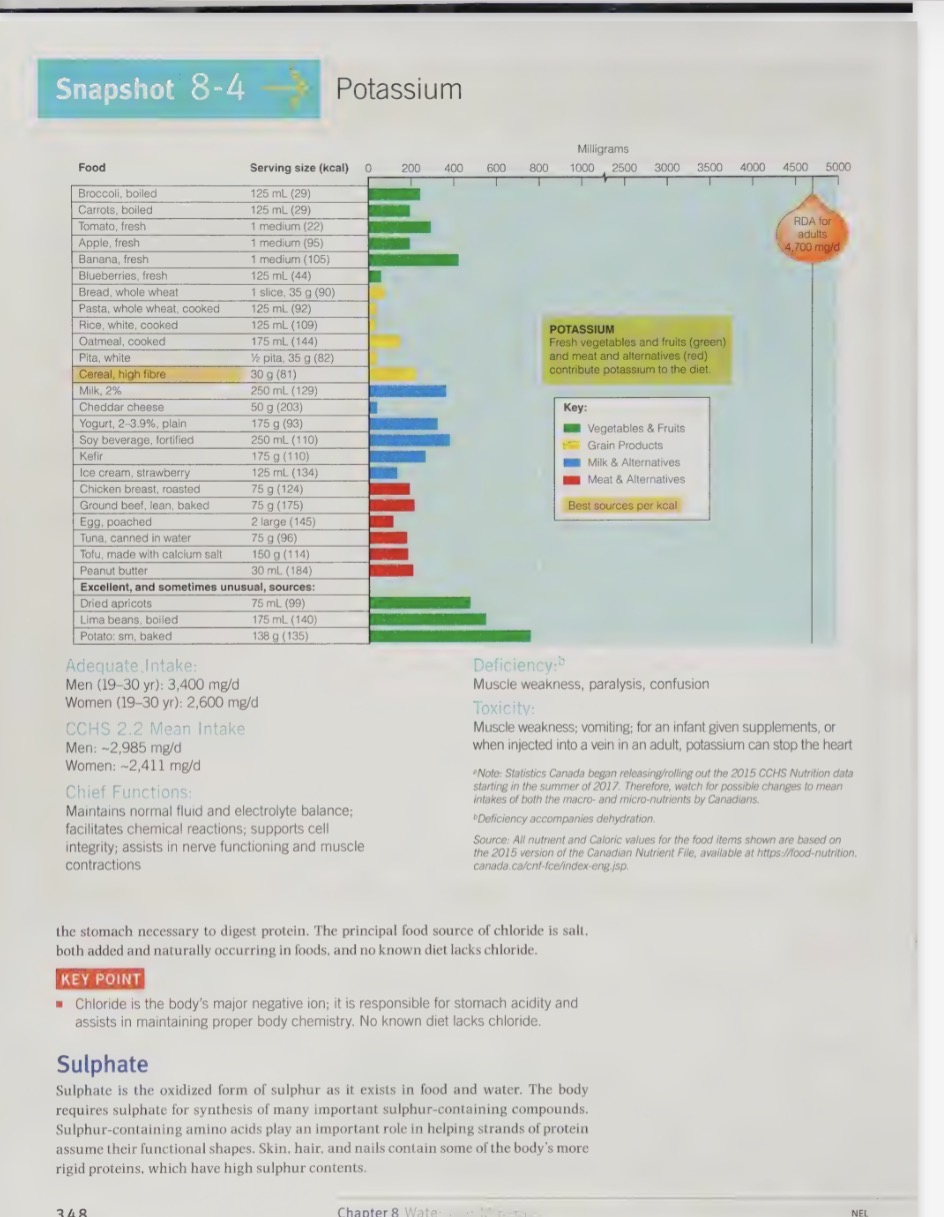
potassium deficiency
accompanies dehydration and cause muscle weakness, paralysis, confusion, and cal also cause death due heart failure
low k intake (below DRI)
worsen hypertension;
cause impaired glucose tolerance;
increase metabolic acidity;
accelerate calcium loss from bones; and
make kidney stone formation more likely.
amount of veggies and fruit recommended by CAD food guide and DASH diet are sufficient to provide the recommended amount of potassium
potassium toxicity
for health individuals, potassium consumed from foods is safe and will not cause toxicity
potassium that is injected into a vein can stop the heart
potassium chloride pills are available over the counter, however should not be used except on physicians advice
oral potassium overdoses are usually not life threatening, because of presence of excess K in the stomach triggers a a committing reflex
a young infant or a person with a weakened heart may not be able to withstand
several infants have died as a result of well-meaning parents overdosing them with K-supplements
other symptoms include muscle weakness and voiding
chloride recommendations
DRI = AI -2,300 mg/d
chloride functions
major negative ion
accompanies sodium in the fluid outside the cells and help to maintain fluid and electrolyte balances and the body’s acid base balance
Cl ion is also part of hydrochloride acid, which maintains the strong acidity of the stomach
chloride food sources
main source of Cl is salt - both the salt added to foods and the alt naturally occurring in food
chloride deficiency
no known diet lacks chloride
chloride toxicity
UL: 3,600 mg/d
toxicity is normally harmless, however can cause vomiting
sulphate
is oxidized form of sulfur found in foods and in the body
body need sulphate for synthesis of many important sulphur - containing compounds, such as the amino acids that form the protein in skin, hair, and nails
these proteins are quite rigid and have high sulphur contents
other important sulphur-containing compounds include antioxidants and the B vitamins biotin and thiamin
DRI recommendations for sulphate
NO DRI recommendations for sulphate and deficiencies are unknown (protein deficiency would occur first, as sulphate is found in all protein contains foods)
sulphate toxicity
can sometimes be seen if dinking water has too much sulphate (either naturally occurring or due to contamination)
this can cause diarrhea and even damage to the colon
iodine (trace minerals) recommendations
although the body only needs a minute amount of iodine (I), it is critical that is obtained
iodine is part thyroxine, which a hormone made by thyroid gland
thyroxine is responsible for regulating the basal metabolic rate
iodine must be available in order of thyroxine to be mad
basal metabolic reactions
The rate at which the body uses energy to support all of the involuntary activities essential for life (e.g., circulation, respiration, etc.).
iodine food sources
essential minerals that support thyroid functions, hormone production, and healthy metabolism
common dietary sources of iodine include:
seaweed, such as nori, kelp, and wakame — these are some of the richest natural sources
fish and seafood, including cod, tuna, shrimp, and other marine foods
dairy products, like milk, yogurt, and cheese, which contain iodine because of animal feed and processing methods
ionizing salt, one of the most common and accessible sources for many people
bread and grain, depending how they’re processed
getting enough iodine is important to prevent deficiencies, which can lead to symptoms like fatigue, weight changes, and thyroid issues
most people meet their iodine needs through a balance diet, but requirements can vary depending on age, pregnancy, and health conditions
iodine deficiency
cells in the thyroid gland enlarge, attempting to trap more particles of iodine
sometimes the cell can grow large enough that they cause a noticeable lump on the neck called goitre
often accompanied b sluggishness and weight gain, because iodine rule is creating thyroxine
woman severely deficient in iodine during pregnancy
this can cause a condition called cretinism (extreme and irreversible mental ad physical impairment) in the infant
much of this can be prevented as long as the deficiency is discovered in the first 6 months of pregnancy
iodine deficiency is the world’s most common and most preventable cause of mental impairment
strategies such as adding iodine to community food or water supplies are underway to attempt to lower incidence of goitre and cretinism
iodine toxicity
UL: 1,100ug/d
excessive intakes of iodine can also cause enlargement of the thyroid gland
iodine is deadly poison when taken in large amounts
iron recommendations
DRI = RDA: men - 8mg/d, women - 18mg/d
Vegetarians: 1.8x DRI
The DRI for women is higher because of menstruation. The DRI committee suggests that vegetarians need 1.8x the amount of Fe recommended for the general population, due to the fact that the iron in plant foods is not as well absorbed as the iron in animal foods.
iron functions
every living cells contains iron (Fe)
most Fe in the body is part of hemoglobin and myoglobin (muscle cells)
helps these proteins to carry oxygen, and release it
also helps many enzymes to use oxygen and is needed to make new cells, amino acids, hormones, and neurotransmitters
fe is stored in the body in the bone marrow and then is sent to the liver where it is packed into new red blood cells which are then shipped off to the blood stream
when red blood cells die, the preen and the liver break them down, salvage the iron and send it back to the bone marrow for storage
iron food sources
meat, fish, poultry,
legumes, eggs
enriched and whole grain products
leafy greens (spinach, swiss chard)
Cast iron pans can transfer Fe to foods in the form of Fe salts. For example, raisins are dried on Fe pans, which increase the Fe content compared to grapes.

iron deficiency
worldwide affects more than 1.2 billion people, and is the most common nutrient deficiency
extreme iron deficiency can result in Fe-deficiency anemia
Fe-deficiency anemia
condition is characterized by red blood cell shrinkage and colour loss due to lower Hb count.
results in reduced delivery of oxygen to tissues, which limits the cells energy metabolism and result sin tiredness, apathy, a tendency to feel cold, irritability, learning disorders, shortened attention span, and clumsiness

slight decrease in Fe levels
can cause fatigue and impact productivity and the capacity to do physicals work
another symptom sometimes seen in people is called pica
pica
A craving for non-food substances such as ice, clay, wall paper paste.