Chem 102 Final Exam Study Guide
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
What is the equation of temperature in Kelvin?
K = °C + 273.15
What is the density formula?
D=mass/volume
What is the isotope formula?
A = number of protons + number of neutrons
Atomic Mass = Σ(fraction of isotope) x (mass of isotope)
What is the Empirical Formula?
Molecular mass = n x empirical mass
How do you find the molarity?
M=moles/Liter
M1V1 = M2V2
How do you find the percent yield?
% Yield = (Actual yield/Theoretical yield) x 100%
What is the Ideal Gas formula?
PV=nRT
D = P * MM /(RT)
How do you find the heat?
Q = mCsDeltaT
What is the formula for finding light?
C = λν
E = hc/λ
What is the order of orbital filling?
1s2s2p3s3p4s3d4p5s4d5p6s
What is the equation for the Formal Charge?
FC = # of valence e- - # of nonbonding e- - ½ # bonding e-
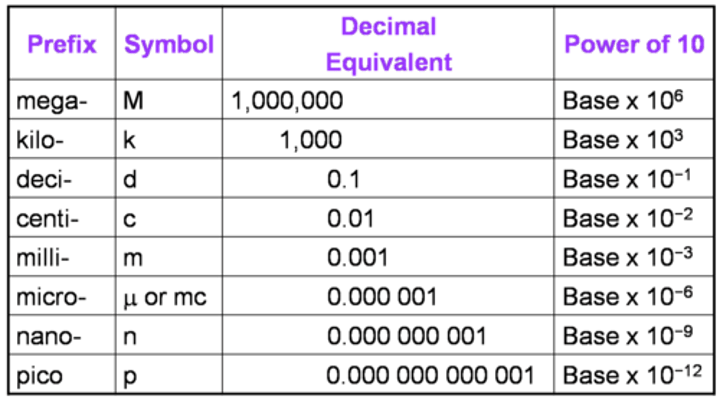
What are the prefix Multipliers?
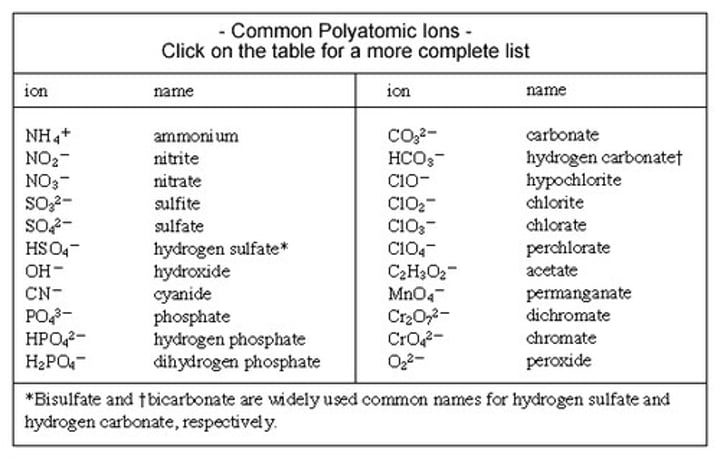
What are the Polyatomic Ions?
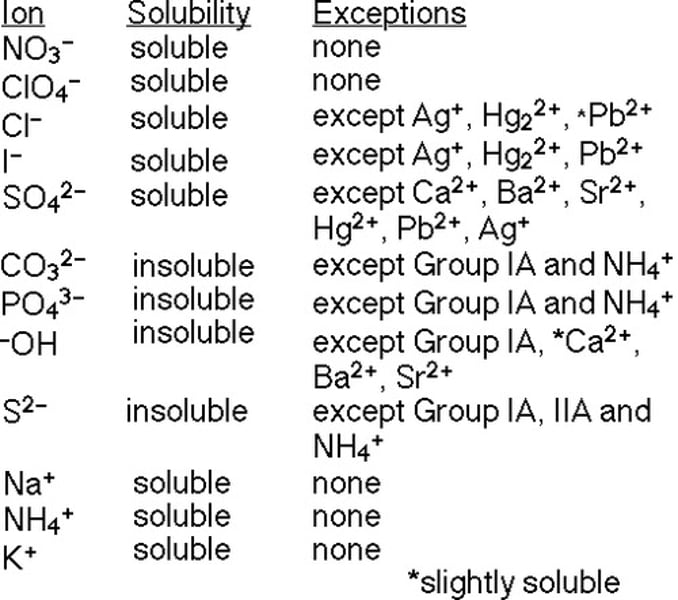
How does the solubility rules work?
How do you draw a Lewis Structure?
Step 1: Count all valance electrons
Step 2: Draw the least electronegative atom in the center with the other atoms spaced evenly around it
Step 3: Draw single bonds to the central atom
Step 4: Complete octets with remaining electrons for the outer atoms first and add remaining electrons (if any) to the central atoms
Step 5: Complete the center atoms octet by making covalent bond(s) using the lone pair electrons from an outer atom of your choice
Step 6: Depending on which electrons you move in Step 5, a variety of resonance structure can be determined
What are the rules of assigning oxidation states?
Step 1: free elements have an oxidation state = 0
Step 2: monatomic ions have an oxidation state equal to their charge
Step 3: (a) the sum of the oxidation states of all the atoms in a compound is 0
(b) the sum of the oxidation states of all the atoms in a polyatomic ion equals the charge on the ion
Step 4:, (a) Group I metals have an oxidation state of +1 in all their compounds
(b) Group II metals have an oxidation state of +2 in all their compounds
5. In their compounds, nonmetals have oxidation states according to the table below
(nonmetals higher on the table take priority)
Prefix Practice: Convert 60 micrometers to millimeters
0.06 Millimeters
Prefix Practice: Convert 30 centimeters to micrometers
300,000 Micrometers
Prefix practice: Convert 80 kilometers to centimeters
8,000,000 Centimeters
Polyatomic ion Practice: What is the formula for Calcium Phosphate?
Ca3(PO4)2
Polyatomic ion Practice: What is the name for this formula: NaC2H3O2
Sodium Acetate
Polyatomic ion Practice: What is the formula be for Copper(II) Nitrate?
Cu(NO3)2
Solubility Practice: Is Calcium Phosphate soluble?
No
Solubility Practice: Is Potassium Chloride soluble?
Yes
Solubility Practice: True or false? Zinc Sulfate is soluble
True, it is soluble!
Oxidation State Practice: What is the oxidation state of Al2O3??
Al = 3+
O = 2-
Oxidation State Practice: What is the oxidation state of HNO2?
H = 1+
N = 3+
O = 2-
Oxidation State Practice: True or false? The oxidation state of PO4^-3 is
P = 5+
O = 2-
True