Topic 2: Intermolecular forces
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
List all the intermolecular and put them in order of strongest to weakest.
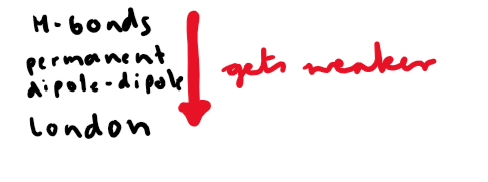
What are forces between? + What are bonds between?

What is another name for London forces?
Also known as instantaneous dipole-induced dipole forces.
When do London forces occur? What type dipole does it cause?
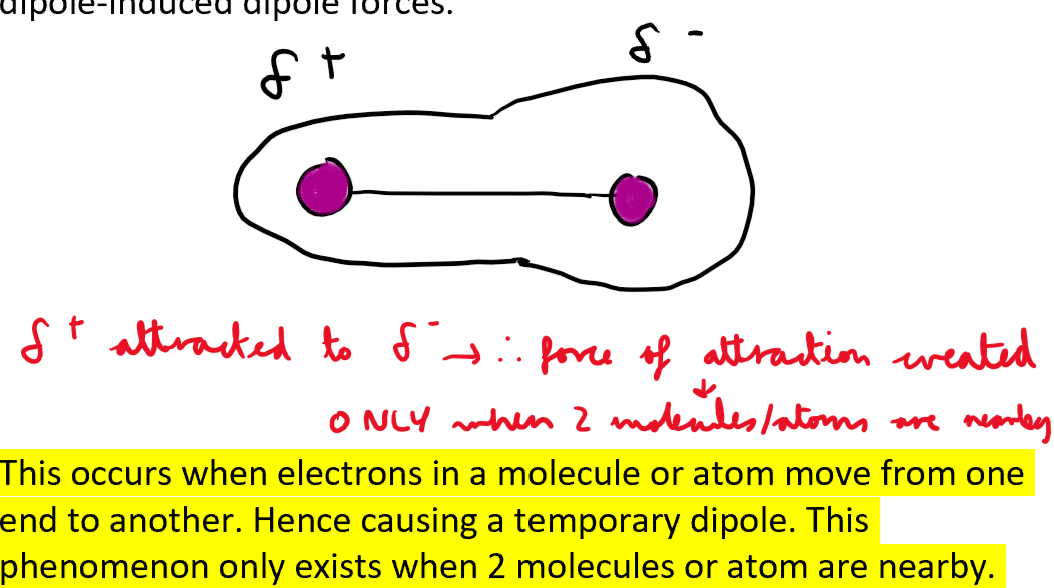
When is the dipole interaction destroyed between 2 molecules/atoms which have London forces between them?
When they move away the dipole interaction is destroyed.
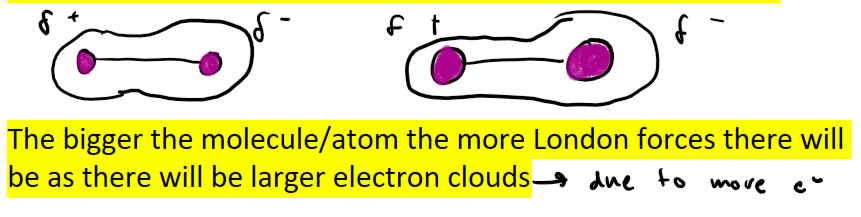
How does the size of a molecule or atom affect the amount of London forces present?
Larger electron clouds due to there being more electrons in the molecules
Out of straight hydrocarbons and branched hydrocarbons. which hydrocarbon has the higher boiling point and why?

Molecules with permanent dipole-dipole interactions also have…?
London forces
In what type of molecules do permanent dipole-dipole interactions exist?
Molecules with polarity
What type of forces exist between molecules with polarity?
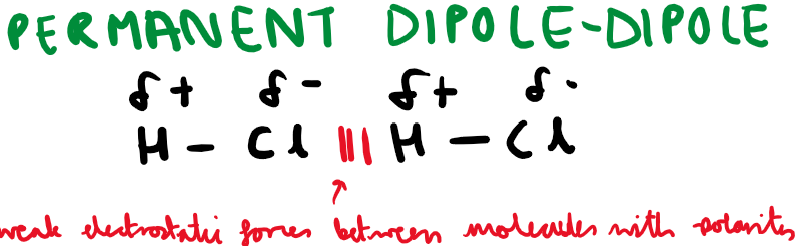
How can you use a charged rod to test for polarity of liquids?
Place charged rod near steady stream of unknown liquid. If liquid bends towards the rod, liquid is polar.
Between which elements does hydrogen bonding occur?
Very electronegative elements, such as H, N, O and F
What is the bond angle of hydrogen bonds?
180 degrees
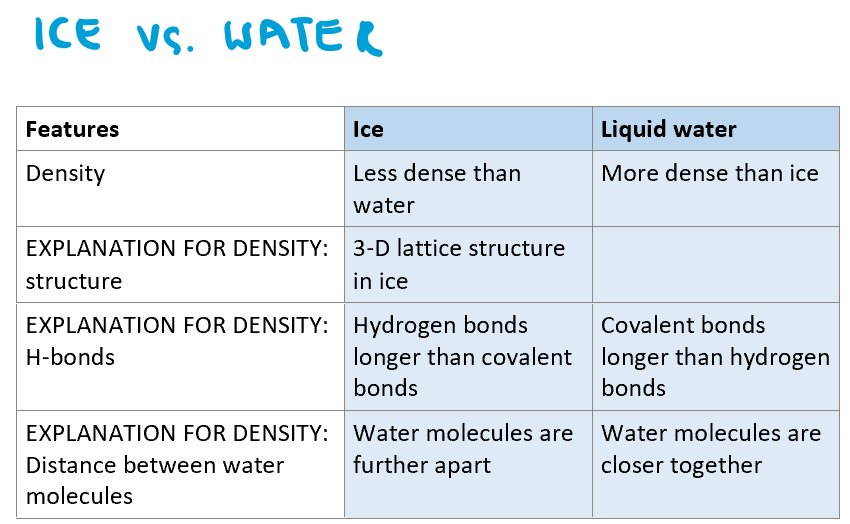
What is more dense: ice or liquid water?
Water
Why is water more dense than ice?