Chemistry - Chapter 2 - Atoms
1/23
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
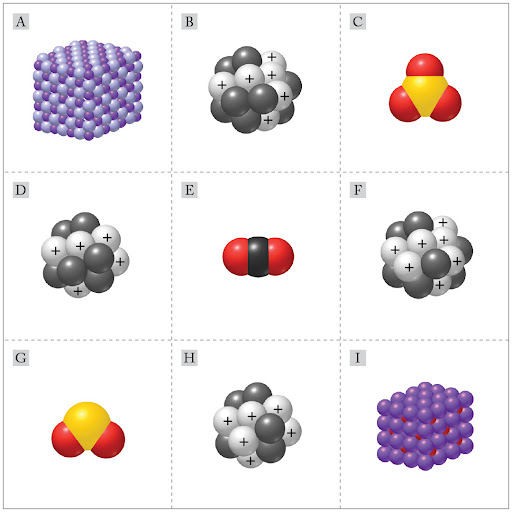
Order the nuclei from the fewest neutrons to the most neutrons.
B < F < D < H
D < B < H < F
H < D < F < B
F < H < B < D
H < D < F < B
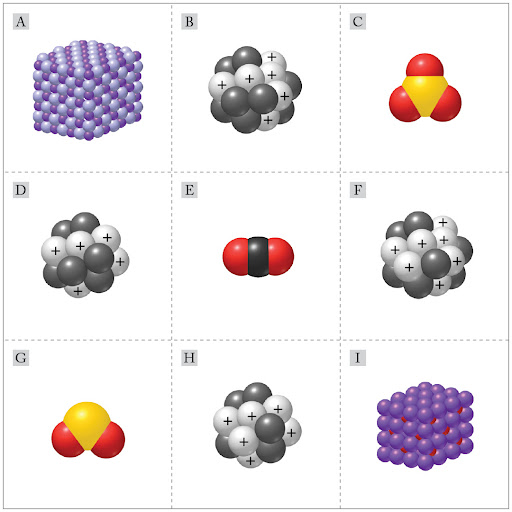
Which representations depict isotopes?
H and F
B, H, and D
B and D
B and H
D and F
B and H
![<p>Which would contain more molecules, 100 g of [C] or 100 g of [G]?</p><p>100 g of [C]</p><p>100 g of [G]</p><p>Both would have the same number of molecules.</p>](https://knowt-user-attachments.s3.amazonaws.com/c8bf341c-6bee-4a44-a18b-ebecfc2d9bf8.jpeg)
Which would contain more molecules, 100 g of [C] or 100 g of [G]?
100 g of [C]
100 g of [G]
Both would have the same number of molecules.
100 g of [C]
Which statement is NOT true concerning cathode rays?
a. They originate from the negative electrode in a cathode ray tube.
b. They travel in straight lines in the absence of electric or magnetic fields.
c. They are made up of electrons.
d. The properties of cathode rays are dependent on the cathode material.
d. The properties of cathode rays are dependent on the cathode material.
Thomson obtained a value of −1.759 × 1011 C/kg (Coulombs/kilogram) for the charge-to-mass (e/m) ratio for the electron. Millikan determined the charge (e) on the electron to be −1.602 × 10−19 C. Calculate the mass of the electron.
1.098 × 1030 kg
9.107 × 10−31 kg
2.817 × 10−8 kg
None of these
9.107 × 10−31 kg
Which of these species has the highest number of electrons?
20Ca
19K+
16S2−
15P3−
20Ca