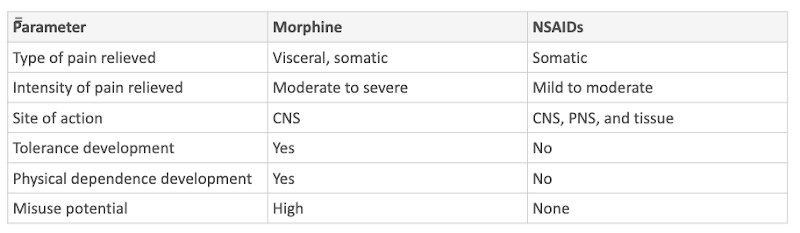
PHAR300 Midterm 2 notes
1/334
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
335 Terms
What is inflammation?
• the immune response to an irritant
• the irritant: germ, a foreign object (splinter), or tissue damage (bone break)
What's the function of an inflammatory response?
- The inflammatory response is a defense mechanism that evolved in higher organisms to protect them from infection and injury.
- Its purpose is to localize and eliminate the injurious agent and to remove damaged tissue components so that the body can begin to heal.
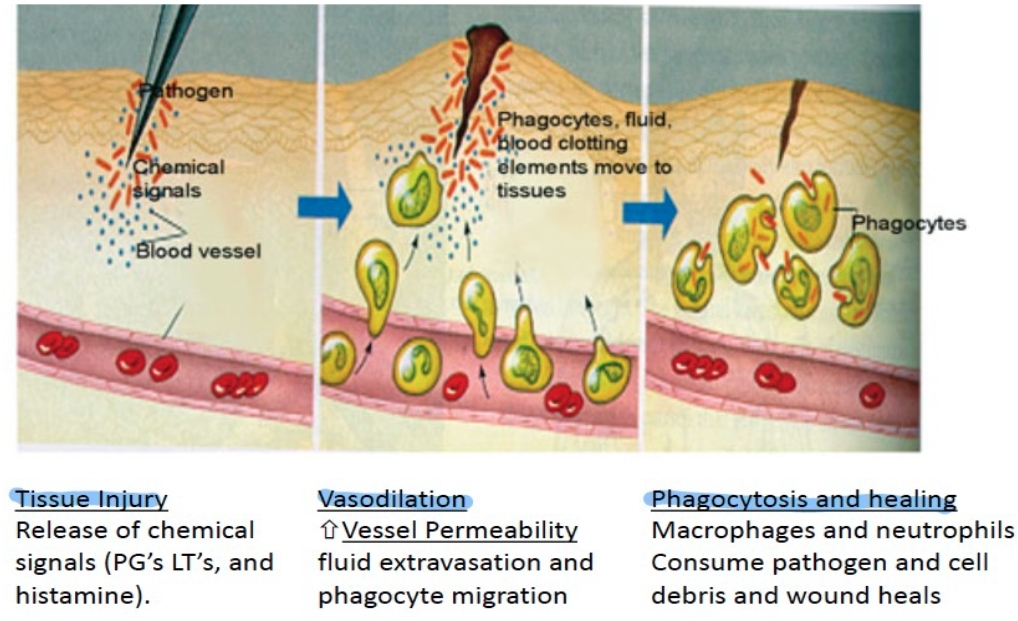
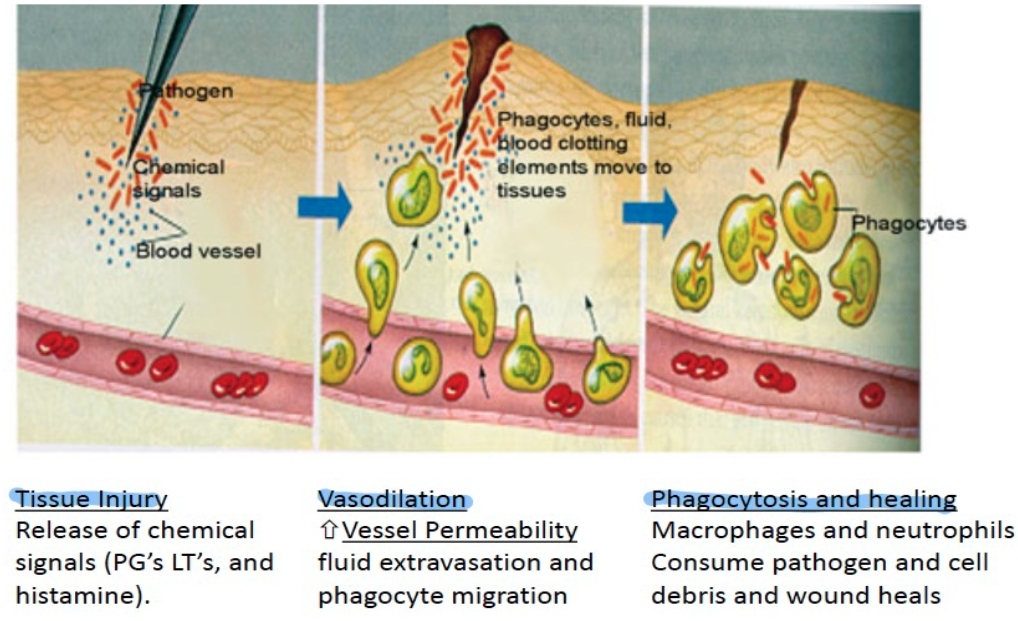
- Tissue injury or infection → vasodilation → increased blood flow and vascular permeability → immune cells and mediators reach the site
- Beneficial process that protects tissue and limits injury, promotes tissue repair and restoration of homeostasis, and helps contain infection.
Biological changes during inflammation?
changes in blood flow, an increase in permeability of blood vessels, and the migration of fluid, proteins, and white blood cells from the circulation to the site of tissue damage.
Common autoimmune diseases

Classification of immunosuppressant drugs

Explain the cells that help heal an injury after a person cuts themselves.
Innate response (PMNs) → APCs → T cells → B cells → Antibodies → Reactivate innate cells
Innate immune response
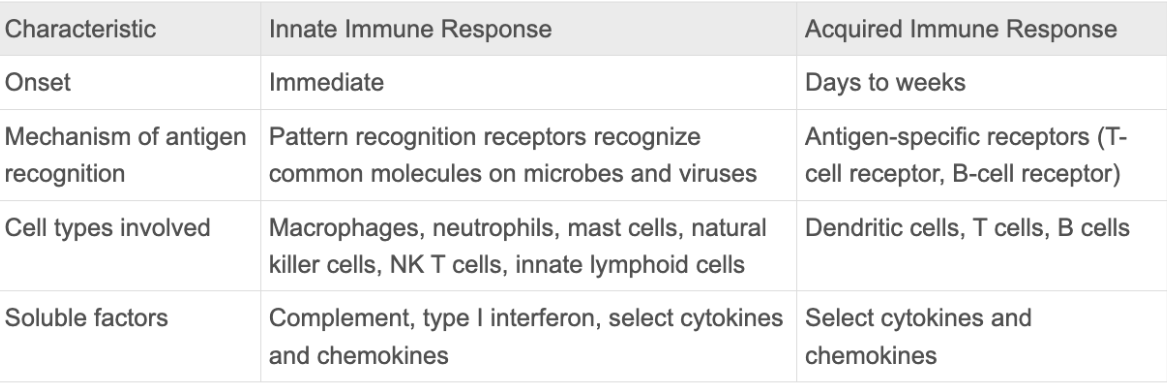
Acquired immune response
Inflammation associated diseases
Causes of unwanted inflammation
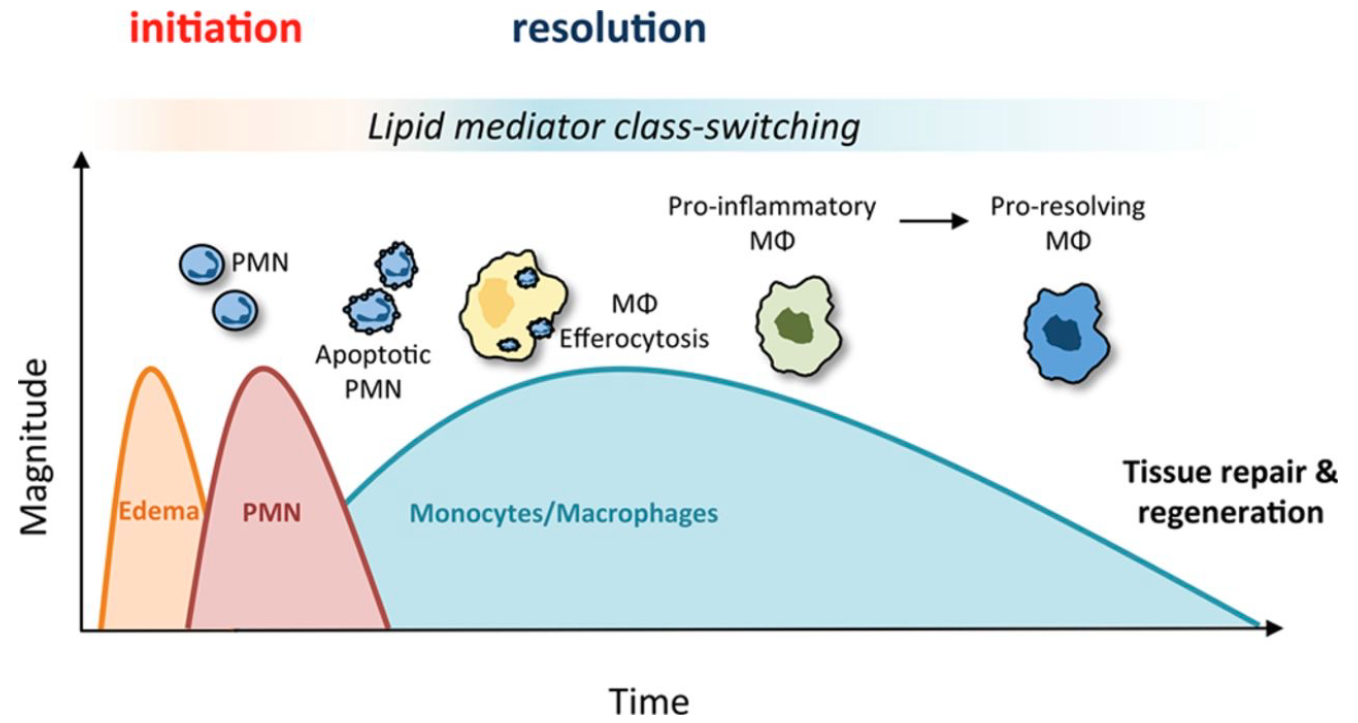
When the inflammation process does not resolve and persists, leading to further tissue damage.
Chronic inflammation leads to auto-inflammatory diseases, where immune cells continuously recruit more cells, resulting in ongoing inflammation and tissue damage.
Anti-inflammatory & Immunosuppressive Drugs
Anti-proliferative drugs
Reduce T cell proliferation → lowers inflammation.
Glucocorticoids (main focus of this lecture)
Broad suppression of the immune system
Immunophilin-binding agents (immunosuppressive drugs)
Antibody-based drugs
Block specific receptors (ex., PD-L1)
When can inflammation be bad?
When antibodies attack self-tissues → autoimmune reaction.
Diagnostic marker: autoantibodies (antibodies recognizing “self”).
How is arthritis caused?
Arthritis: Autoantibodies cause chronic inflammation → tissue destruction (esp. in joints/synovial fluid).
How is asthma caused?
Body overreacts to harmless triggers (allergen, cold air) and the immune system causes inflammation → Mucus production → Airway constriction
What are Corticosteroids? (function?)
They are a class of drug that lowers inflammation and immune system activity in the body.
Turn off the immune system: immunosuppressive therapy
Under what conditions are Corticosteroids prescribed?
Because corticosteroids ease swelling, itching, redness, and allergic reactions, doctors often prescribe them to help treat diseases like asthma & arthritis.
Non-steroidal anti-inflammatory drugs (NSAIDs)
are medicines that are widely used to relieve pain, reduce inflammation, and bring down a high temperature.
NSAID vs Corticosteroids: when are they used?
NSAID for acute, Corticosteroids for chronic
What is a steroid?
Biologically active organic compound with four rings (can be different in different steroids) arranged in a specific molecular configuration. Made from cholesterol.
What are the principal functions of a steroid
1.) Important components of cell membranes, which can alter membrane fluidity,
2.) Are signalling molecules
What does “Corticosteroids” refer to?
In technical terms, "corticosteroid" refers to both glucocorticoids and mineralocorticoids (as both are mimics of hormones produced by the adrenal cortex), but is often used as a synonym for "glucocorticoid".
Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances (electrolyte balance and fluid balance). The primary mineralocorticoid is aldosterone
Before we turned them into a drug, what is the role of steroids in our body?
Our body produces glucocorticoids as a response to stress (to reduce our inflammatory responses)
Endocrine system
Complex network of glands and organs.
Uses hormones to control and coordinate your body’s metabolism, energy level, reproduction, growth and development, and response to injury, stress, resolution of inflammation (reduces inflammation, swelling at the end) and mood
What is the signalling pathway in the kidney that produces glucocorticoids
Hypothalamus → releases CRH (Corticotropin-Releasing Hormone) + AVP (less important) on pituitary
Anterior Pituitary → releases ACTH (Adrenocorticotropic Hormone) on adrenal gland
Adrenal gland → releases glucocorticoids (cortisol).
↑ Cortisol levels → signal hypothalamus & pituitary to stop producing CRH & ACTH.
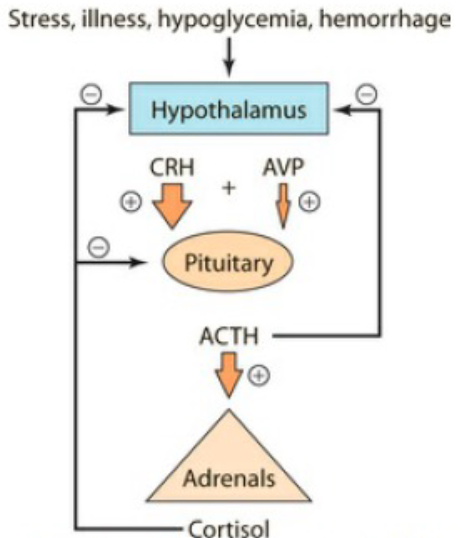
Explain the mechanism in the kidney that produces glucocorticoids
CRH (41-aa peptide) is a prohormone produced in the hypothalamus
CRH binds a G protein–coupled receptor in the pituitary → activates POMC
POMC (proopiomelanocortin) expression → ACTH synthesis.
ACTH travels from pituitary → adrenal gland (on top of kidneys).
ACTH function?
Regulates glucocorticoid synthesis in the zona fasciculata / reticularis (zone of adrenal cortex)
Increases the delivery of cholesterol to the inner mitochondrial membrane
Increases transcription of steroidogenic enzymes
Zona fasciculata / reticularis function?
Zone of the kidney
Produces glucocorticoids
Key enzymes in this zone: CYP17 & CYP11B1 are both crucial for cortisol production.
CYP17 & CYP11B1 function?
Enzymes in the Zona fasciculata / reticularis that’s crucial for cortisol production
Explain the process of how a Cholesterol molecule turns into a cortisol
i) Cholesterol = starting molecule.
ii) Converted to pregnenolone or progesterone.
iii) Modified by CYP17 and CYP11B1 → forms cortisol.
Exogenous steroid drugs (like prednisone) affect on cortisol production?
Less natural cortisol production and stimulates the negative feedback effect
Long-term high-dose exogenous steroid leads to shutdown of HPA axis and adrenal atrophy (uncommon with low or medium doses)
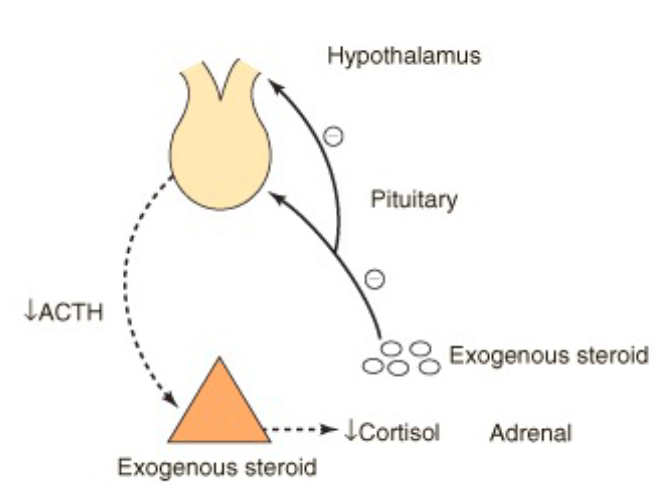
What is the natural production of corticosteroids induced by?
Stress
Can cortisol (glucocorticoid) travel alone in the blood?
No, it must bind to CBG
CBG function?
Transports glucocorticoids in the blood
Keeps it inactive and serves as a reservoir (until stress response)
Does a low CBG affect glucocorticoid therapy?
Yes, because it’s unable to carry it through the blood & less of a reservoir
How does stress/inflammation affect glucocorticoids in the blood?
Inflammation, stress, induced release of molecules like elastase release the steroid from the complex
Explain the mechanism of action for steroid hormones (e.g. glucocorticoids)
Intracellular receptors (typically cytoplasmic or nuclear)
Glucocorticoids are lipid-soluble → cross cell membrane.
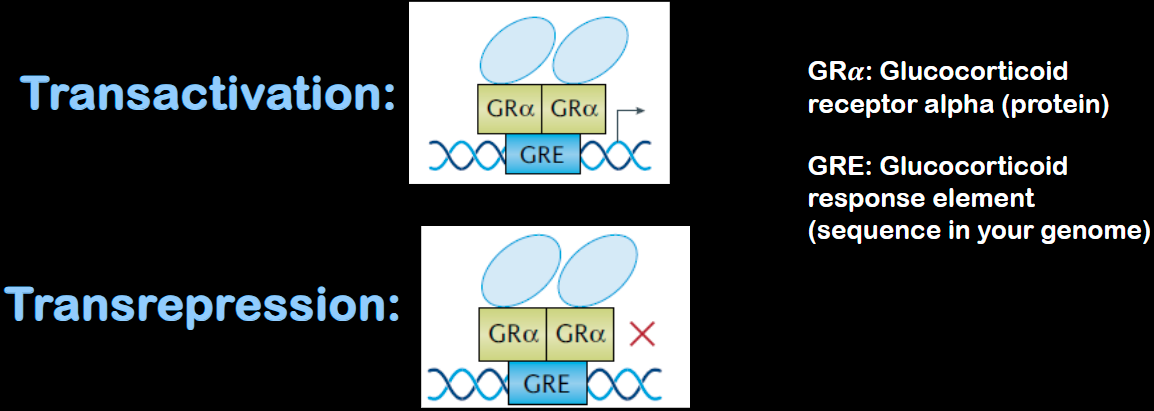
In nucleus: acts as a transcription factor → Activates or represses specific genes
Initiate signal transduction for steroid hormones, which lead to changes in gene expression over a time period of hours to days.
How do glucocorticoids regulate gene expression?
A chaperone complex helps transport GR–hormone complex into nucleus, where it can turn on/off different genes
Bind to glucocorticoid receptor (GRα) inside cytoplasm.
GRE: Glucocorticoid response element (sequence in your genome)
Addison's disease
When the adrenal glands don't make enough cortisol and aldosterone (rare disease)
Causes hypoglycemia, bone issues, and weight loss (giving glucorticoids restores metabolism)
Addison's disease treatment
Hydrocortisone (Cortef), prednisone or methylprednisolone to replace cortisol. These hormones are given on a schedule to mimic the normal 24-hour fluctuation of cortisol levels.
Fludrocortisone acetate to replace aldosterone.
Absorption of glucocorticoids (pharmacokinetics)
Absorbed rapidly and readily from the GI tract and from synovial and conjunctival spaces due to their lipophilic character, but are slowly absorbed from the skin.
How is glucocorticoid absorption disrupted?
Disrupted by concentrations of estrogen (pregnancy, contraceptive use, or hormone replacement therapy), requiring increased plasma cortisol concentrations to maintain an appropriate bioactive fraction
How are glucocorticoids distributed? (pharmacokinetics)
Circulating cortisol is 80%–90% bound to plasma proteins with high affinity to corticosteroid-binding globulin (CBG, transcortin),
5%– 10% loosely bound to albumin
3%–10% as the free, active fraction.
CBG can also bind synthetic glucocorticoids such as prednisone and prednisolone, but not dexamethasone, resulting in almost 100% of plasma dexamethasone bioactive.
Metabolism of glucocorticoids
Glucocorticoids are primarily metabolized in liver through enzymatic reactions that convert them into inactive, more water-soluble metabolites: tetrahydrocortisol (THF) and tetrahydrocortisone (inactive)
Glucocorticoid elimination
Tetrahydrocortisol-glucuronide and tetrahydrodeoxycortisol-glucuronide are then conjugated to glucuronic acid or sulfate before being excreted in the urine.
This process reduces the physiological activity of the steroid and allows for its elimination
How do we increase the half-life (i.e. metabolism) of glucocorticoids
Addition of a fluorine atom at position 9 = enhances glucocorticoid activity (only activity)
Addition of a methyl group at position 16, as present in betamethasone and dexamethasone = increases receptor activation + increases the duration of action of these compounds (duration + activity)
What are the 3 main therapeutic uses of glucocorticoids
(1) replacement therapy for patients exhibiting inadequate endogenous cortisol production
(2) anti-inflammatory or immunosuppressant agents;
(3) adjuvants in the treatment of myeloproliferative diseases and other malignant conditions.
How do glucocorticoids help with asthma
Used as inhaled anti-inflammatory glucocorticoids (usually inhalers)
Reduce lung inflammation and mucus production.
Often combined with other asthma medications.
What is EVALI?
E-cigarette or Vaping Product Use–Associated Lung Injury:
Reported mainly in 2019 outbreak (non-infectious).
Caused by vaping THC and vitamin E acetate.
Led to severe lung inflammation in some teens.
How is EVALI treated?
Glucocorticoids MAY reduce inflammation and help lungs heal.
Metabolic Effects of Glucocorticoids
Generally, transcriptional activation which can alter metabolism (especially with long-term use)
Increase blood glucose to protect the brain/heart (hyperglycemia).
↑ glycogenolysis, gluconeogenesis, lipolysis, protein catabolism + ↓ protein synthesis
Myeloproliferative disorders
• Types of blood cancer that begin with an abnormal mutation (change) in a stem cell in the bone marrow
• Changes lead to an overproduction of any combination of white cells, red cells and platelets
Treatments to Myeloproliferative diseases? Can it be cured?
Glucocorticoids are used to reduce excessive blood cell production
Treatment aims to correct abnormal blood counts, but myeloproliferative neoplasms cannot be cured
In some cases (angiogenic myeloid metaplasia), glucocorticoids may increase RBC lifespan.
In autoimmune patients, why do autoimmune patients have increased inflammation at night? Why are they more sensitive to glucocorticoid drugs at this time?
Glucocorticoids naturally follow a circadian rhythm, with levels peaking during the day and reaching their lowest point at night. This makes timing an important factor in treatment, as both the effectiveness of the drug and the likelihood of side effects can vary depending on when it is taken (important in personalized medicine).
In autoimmune patients, inflammation often worsens at night when glucocorticoid levels drop. Also, at these low points, the body is more sensitive to the effects of the drug (at high points, we are more resistant).
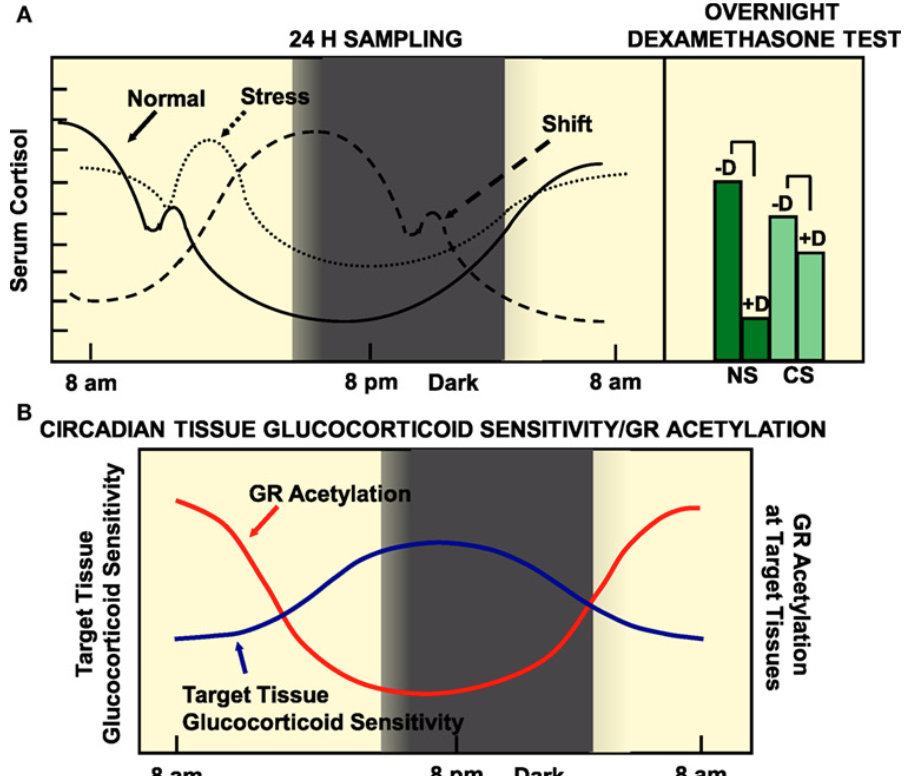
Major Adverse effects of short-term glucocorticoid treatment
Muscle deterioration, bone growth inhibition (especially in children), depression or mental illness, skin issues (acne, thinning), diabetes and metabolic disturbances, increased infection risk
Adverse effects of prolonged glucocorticoid treatment
o Osteoporosis and fracture (> or =3 months: increase in fracture risk)
o Glucose intolerance and diabetes (doubled in rheumatoid arthritis patients taking 7.5 mg or + prednisone)
o Central obesity
o Muscle wasting
o Increased risk of infections
o Depression
o Cataracts
Adverse effects of glucocorticoids (even with local administration?)
● Oral candidiasis (thrush)
o Yeast growing on the tongue due to immunosuppression (usually inhaler users).
o Can be avoided by rinsing mouth after using inhaler to remove glucocorticoids from the mouth to prevent shutting down the immune responses there.
● Growth inhibition
o Pre-pubescent patients are at risk, so it is important to have the right dose in inhalers (for ex) for kids to keep the glucocorticoids in the lungs and prevent systemic effects.
● Decreased bone density
HPA axis suppression
● Oral corticosteroids (ex,. Prednisone)
o Extreme cases
o Short duration
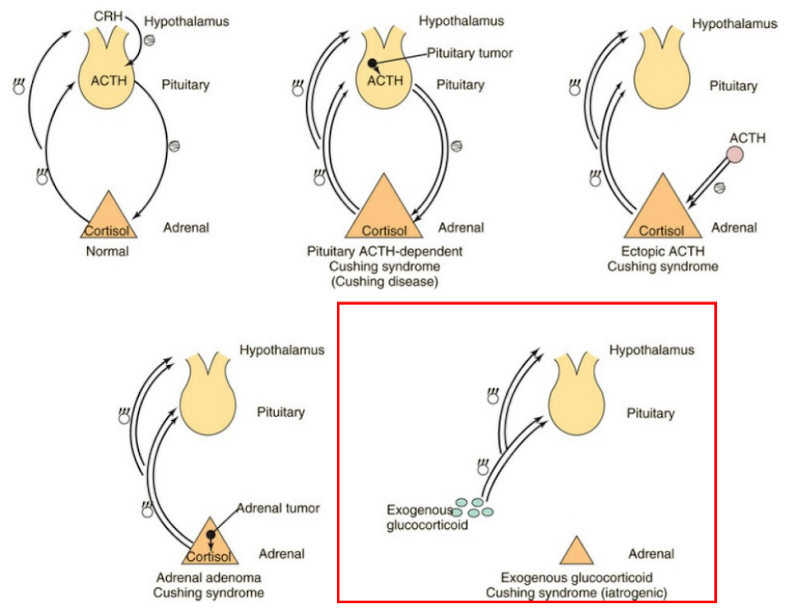
Cushing’s syndrome
Excess Glucocorticoids:
“Moon face” (round, puffy face) + Fluid retention
Fat accumulation in certain body areas + Changes in glucose metabolism
Possible psychiatric issues
What are the 4 main causes of Cushing’s disease?
Pituitary tumor:
Overproduces ACTH → constant adrenal stimulation
Negative feedback fails → so high cortisol continues
Ectopic ACTH production (ex, small cell lung cancer):
ACTH produced outside pituitary
Stimulates adrenal gland → high cortisol
Adrenal tumor:
Tumor in adrenal gland → overproduction of cortisol
Independent of ACTH or CRH
Excess exogenous glucocorticoids (red box):
Long-term steroid use → oversaturation → same symptoms
History of NSAIDs (low weight)
1828: White willow (Salix alba) and Meadowsweet (Spirea ulmaria) plant extracts were found to contain a compound that is effective in treating pain and inflammation.
1897: Therapeutic compound identified as salicylic acid → used to synthesize acetylsalicylic acid (ASA), which has fewer side effects → creation of aspirin.
Aspirin name origin: a – acetyl; spir – spirea (plant); in – common suffix for “drug” in the 1800s
The acetyl group added to salicylic acid improves the binding selectivity of the drug, causing fewer side effects.
1899: Aspirin patented by Bayer. Drug first released to the public.
1971: Mechanism of action of ASA discovered by Sir John Robert Vane, an English pharmacologist
ASA blocks the synthesis of prostaglandins (PGs)
History of Acetaminophen (low weight)
1852: Discovery of acetaminophen, originally called acetanilide. Physicians noticed it reduced fever in sick kids.
1899: Karl Morner discovered the relationship between acetanilide and acetaminophen, identifying acetaminophen as the active metabolite responsible for the therapeutic effects.
1909: Joseph Freiher von Mering confirmed the drug’s effectiveness against pain and fever but recommended further study.
1949: Acetaminophen was reintroduced and recognized as a safe and effective analgesic and antipyretic. Modern clinical trials confirmed its utility.
1950s–present: Marketed under the brand name Tylenol (McNeil Laboratories). Later formulations combined acetaminophen with other active ingredients (ex.: Tylenol PM, Tylenol Cold & Flu).
Note: The exact mechanism of action of acetaminophen remains unclear, though it is thought to act centrally.
Acetaminophen (tylenol) function?
Mechanism not entirely understood, but behaves like a weak COX inhibitor, with evidence for preferential COX-2 inhibition in the CNS and/or activity at COX splice variants.
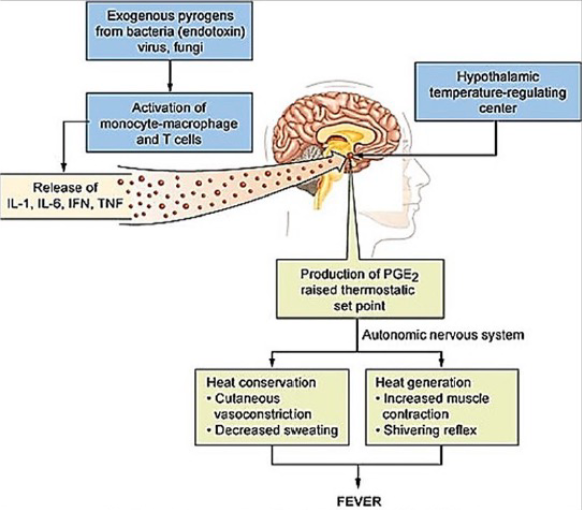
What are the 5 cardinal signs of Inflammation?
Fever
Redness
Swelling
Pain
Loss of function
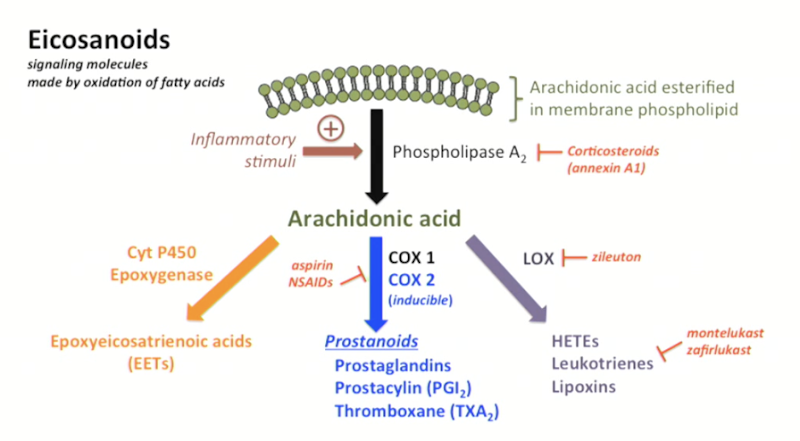
What are Eicosanoids?
Soluble signaling molecules made by oxidation of fatty acids.
Principal mediators of the inflammatory response.
Recruit more immune cells + promote cytokine release
Effects of prostaglandins
Prostaglandins interact with various GPCRs to produce biological effects
Renal homeostasis
Vasodilation and vasoconstriction
Platelet aggregation/inhibition (control coagulation within the circulation)
Blood flow to different organs by controlling vasoconstriction and vasodilation
Amplified pain and inflammation response
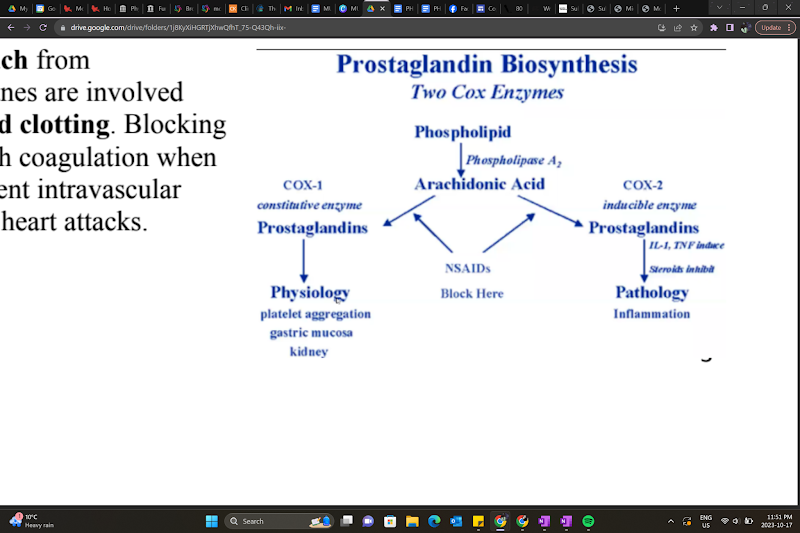
NSAIDs function?
inhibit COX-1 and/or COX-2, reducing prostaglandins → less inflammation, fever, and pain.
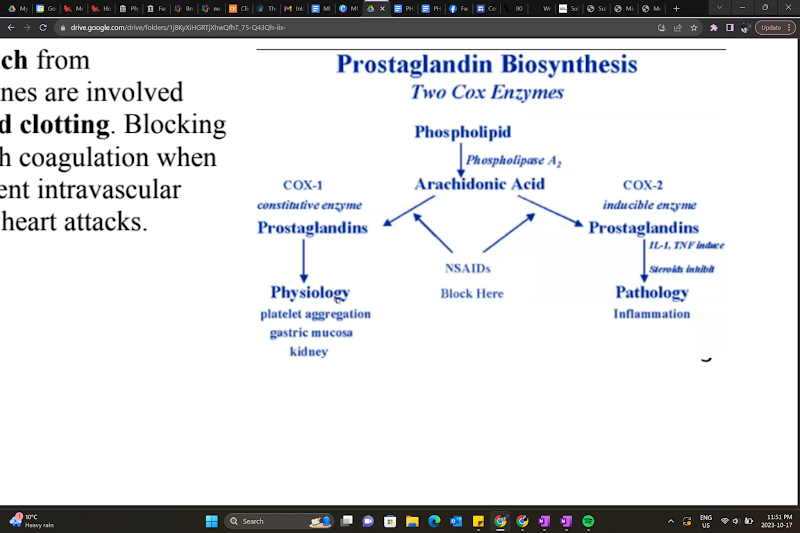
Prostaglandin (Eicosanoids) synthesis
A section of the plasma membrane can be ‘clipped off’ by phospholipase A2 → arachidonic acid release
Arachidonic acid is a substrate for many biological products, producing different biologically active compounds (ex.: acting on COX enzymes)
COX-1 and COX-2 (cyclooxygenases) produce prostanoids
Products are tissue-specific.
Ex.: TXA2 from platelets, PGI2 from endothelium
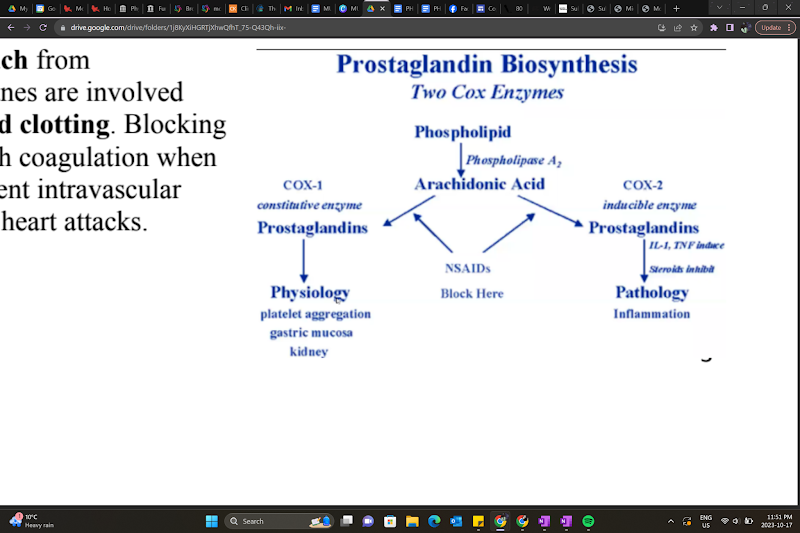
What are some side effects of NSAIDs regarding prostaglandin synthesis?
Prostaglandins protect the stomach from hydrochloric acid, and thromboxanes are involved in platelet aggregation and blood clotting. Blocking prostaglandins could interfere with coagulation when you need it, but it could also prevent intravascular coagulation and protect you from heart attacks.
Acetaminophen
Shares analgesic and antipyretic activity with the
NSAIDs but lacks other actions associated with
COX inhibition.Weak inhibitor of COX enzymes
Preferentially inhibit a COX splice variant
expressed in the brain, which could explain its
anti-inflammatory effects in peripheral tissues.
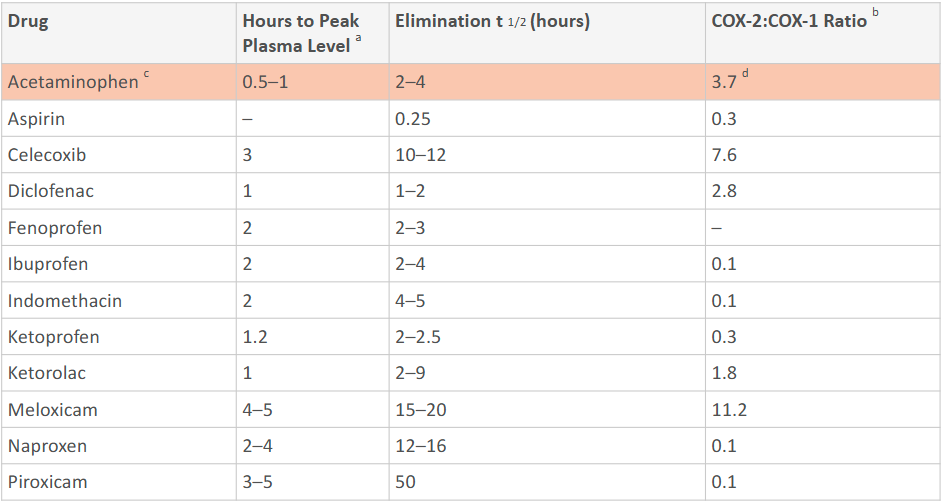
Is the COX-2:COX-1 ratio of NSAIDs high or low? How about acetaminophen? How does the side effects differ?
Low: Aspirin has more gastric side effects because it inhibits COX-1.
High: Acetaminophen produces minimal gastric side effects
Is Acetaminophen a NSAID (anti-inflammatory agent)? Why?
No, because it primarily acts on the brain with little effects on the rest of the body
Are the function of COX enzymes tissue specific?
Yes
Peripheral Sensitization
Pain sensitization, or central sensitization, is a state where the central nervous system becomes hypersensitive and amplifies pain signals, even without a recent injury.
Explain the process of how nociceptors are activated and send signals to the brain
When tissue is damaged, cells and immune mediators (macrophages, mast cells) release signaling molecules such as prostaglandins (PGs), bradykinin, and serotonin (5-HT).
These mediators sensitize nociceptive nerve endings, lowering their threshold for activation (they fire more easily in response to pain stimuli)
Result: increased frequency of action potentials from the site of injury to the spinal cord and then to higher brain centers, where pain is perceived.
Prostaglandins role in pain?
PGs DO NOT directly cause pain, but they amplify pain signaling by sensitizing nociceptors to other mediators.
This is why inflammation makes normally mild stimuli feel painful. Nociceptors have become hypersensitive.
NSAIDs role in pain?
NSAIDs act at the periphery, blocking COX enzymes → less PG synthesis
With fewer PGs available, nociceptor sensitization decreases, reducing the intensity of the pain signal reaching the spinal cord and brain.
NSAIDs are therefore more effective when treating mild to moderate pain caused by inflammation or tissue injury
What’s an example of using NSAIDs to treat pain?
Aspirin is one of the most commonly used agents in treating headaches and mild to moderate pain arising from muscles, tendons, and joints.
Opioids vs NSAIDs to treat pain
Compared to opioids, NSAIDs are much less effective in treating visceral pain, and severe pain. However, there is no potential for analgesic tolerance or physical dependence. NSAIDs are not addictive.
Acetaminophen role in pain?
Acetaminophen is a popular alternative for treating pain, but it is less effective in treating pain caused by inflammation.
Analgesia and antipyretic effects are mediated by the CNS, but the mechanism of action is unknown.
Combination drugs (NSAID/acetaminophen + opioid analgesic): Increase in analgesic efficacy compared to NSAIDs or acetaminophen alone
What are platelets?
Platelets are small, colorless cell fragments without nuclei, produced in the bone marrow. They circulate for about 10 days and are essential for blood clotting (when activated).
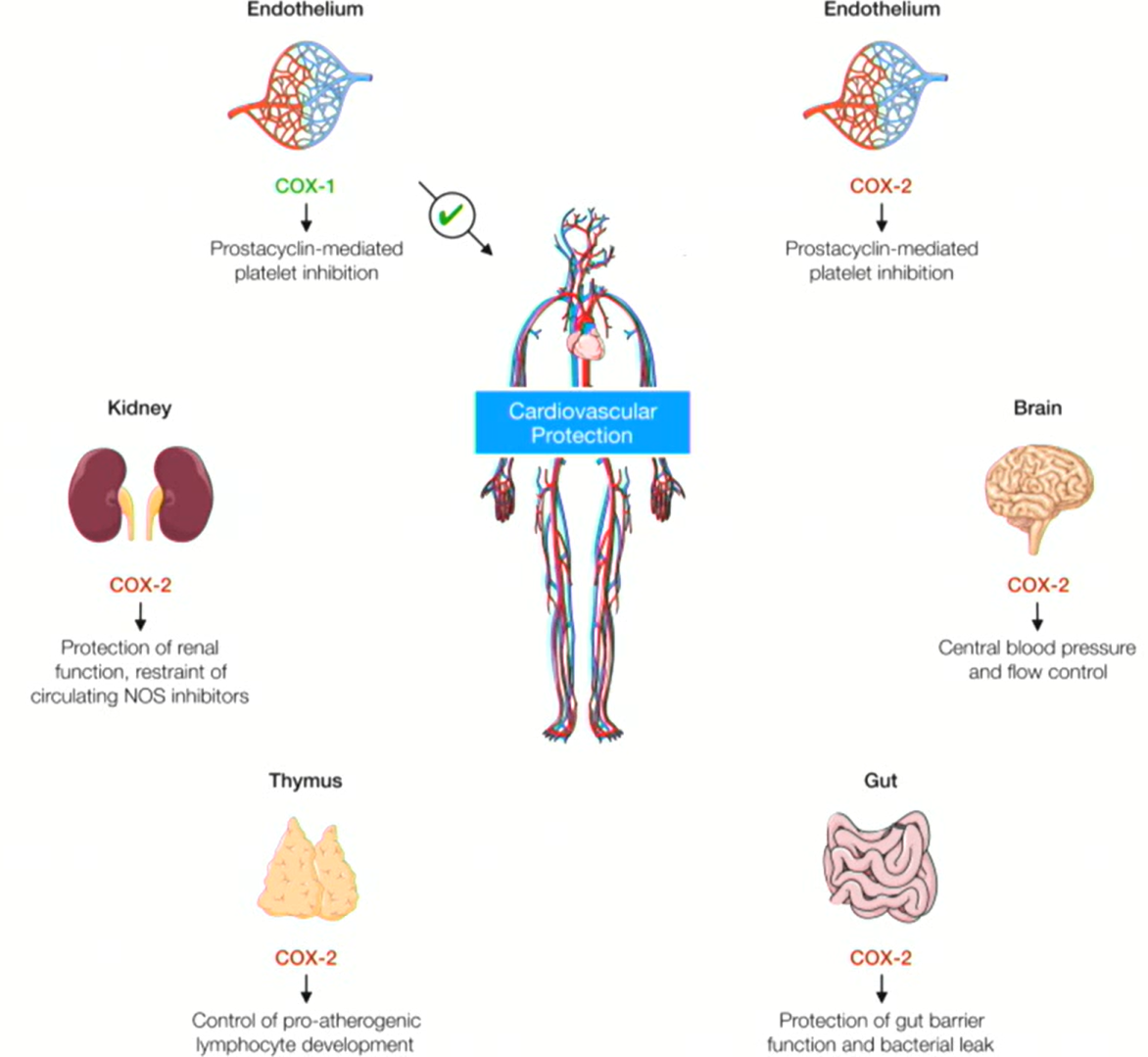
COX-1 function in platelets?
COX-1 in platelets produces thromboxane A2 (TXA2)
→ causes vasoconstriction and platelet aggregation
COX-2 function in endothelial cells?
COX-2 in endothelial cells produces prostacyclin (PGI2)
→ causes vasodilation and inhibits platelet aggregation
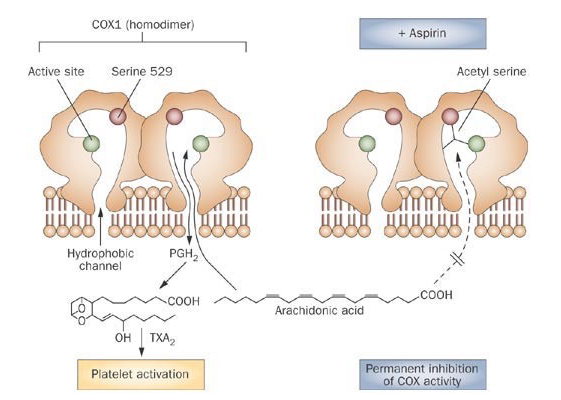
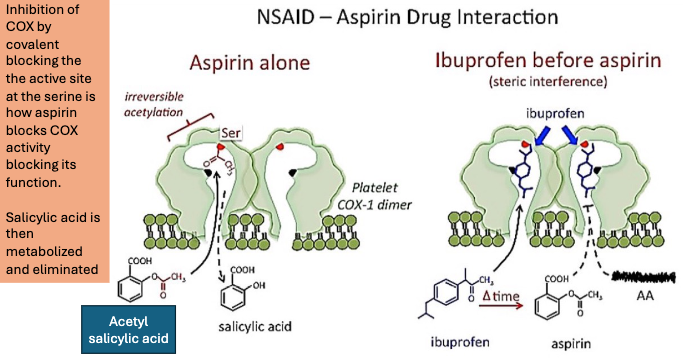
Aspirin effect on COX?
Aspirin irreversibly inhibits COX-1, blocking TXA2 formation in platelets.
Aspirin reversibly inhibits COX-2
Since platelets don’t have nuclei, they cannot resynthesize TXA2 → the effect lasts the entire platelet lifespan.
Endothelial cells can regenerate COX-2, so their vasodilatory function recovers quickly.
How does Aspirin inhibit COX?
Aspirin inhibits COX by covalent acetylation
In what conditions do we administer a low dose of aspirin?
a low dose of aspirin can be used to reduce the risk of recurrent myocardial infarction, stroke, and vascular death.
Aspirin inhibits COX-1 (constriction) and COX-2 (dilation), but COX-2 can regenerate
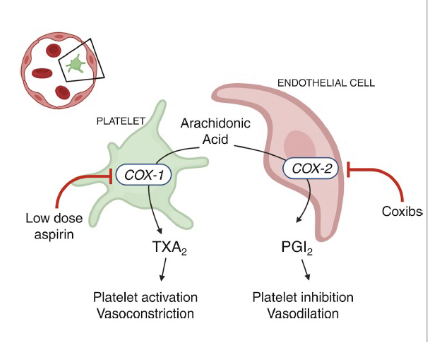
In what conditions do we administer a high dose of aspirin?
Pain and inflammation (blocks both COX-1 and 2)
Why can platelets not regenerate COX enzymes, whereas endothelial cells can?
Because they lack nuclei
Non-selective NSAIDs pharmacodynamics? Examples?
Act on both COX-1 and COX-2 enzymes.
Reversible, competitive inhibition.
Analgesic and anti-inflammatory effects, but can also reduce protective PGs in the gut (GI side effects)
Ex.: Ibuprofen, Naproxen, Diclofenac
COX-2 selective inhibitors pharmacodynamics? Examples?
Act on COX-2 only
Developed to spare COX-1, reducing GI side effects
Canines and some other species are highly sensitive to COX-1 inhibition, so veterinary NSAIDs are designed to be COX-2 selective to reduce toxicity
Ex.: Celecoxib
Ibuprofen pharmacodynamics?
Reversible, competitive inhibitor: Competes with arachidonic acid at the COX active site.
Effect stops once it is cleared → temporary
Aspirin pharmacodynamics?
Irreversible, non-competitive inhibitor.
Covalently acetylates a serine residue in the COX active site, permanently inactivating the enzyme.
In platelets, effect lasts for the full platelet lifespan (~10 days) → long-lasting
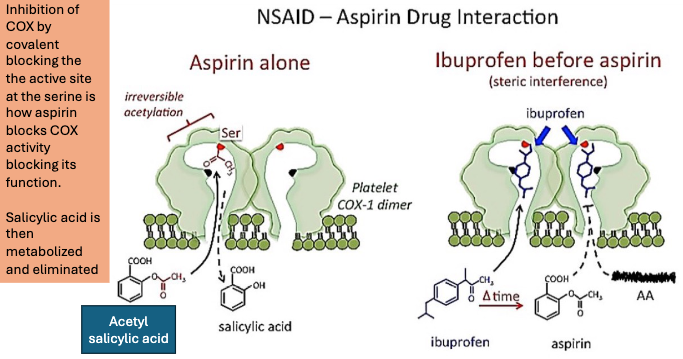
How does Ibuprofen impact the effectiveness of aspirin?
Ibuprofen then aspirin:
If ibuprofen is taken before aspirin, it occupies COX-1 reversibly, blocking aspirin’s access to the active site, until ibuprofen dissociates.
This prevents aspirin from acetylating the enzyme → reduces aspirin’s antiplatelet effect.
BUT: Regular use of ibuprofen can diminish aspirin’s cardioprotective effects.
NSAIDs: General absorption properties
All NSAIDs are weak acids with low pKa values → efficient absorption from the acidic stomach and duodenum
Rapid and complete absorption with oral administration
Aspirin absorption properties
Enters the plasma within ~15 mins following ingestion
Rapidly hydrolyzed by esterases in the stomach, plasma, and tissues → salicylic acid (active metabolite)
Salicylic acid plasma half-life varies with dose
Doses used to treat pain or fever: T1/2 = ~2-3 hours
Doses used to treat inflammatory disorders: T1/2 up to 12 hours (switches to zero-order kinetics)
Peak salicylate concentration depends on dose
High bioavailability, but may vary with formulation
NSAID (aspirin) distribution
Rapid distribution throughout the body
Binding to plasma proteins (albumin) creates the potential for interactions with other drugs that also bind to plasma
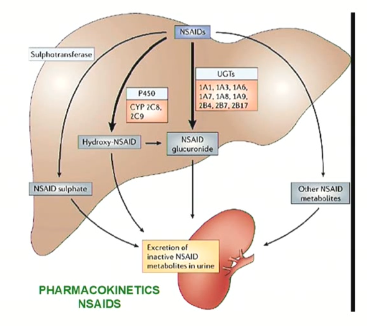
NSAID (aspirin) metabolism
Primarily occurs in the liver, where P450 enzymes and other metabolic pathways convert NSAIDs to inactive metabolites:
Phase I
Naproxen and indomethacin are demethylated prior to conjugation and excretion
Piroxicam and fenoprofen are hydroxylated prior to conjugation and excretion
Ibuprofen is hydroxylated and carboxylated prior to conjugation and excretion
Phase II
~75% conjugated to glycine in the liver
→ forms inactive salicyluric acid
This metabolite is excreted by the kidneys alongside glucuronide conjugates and 10% free salicylic acid
Due to the limited hepatic pool of glycine and glucuronide available for conjugation, salicylate is eliminated by first-order kinetics at lower doses, and by zero-order kinetics at higher doses.
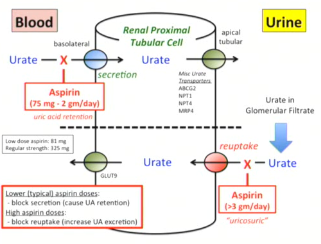
NSAID elimination
Quick elimination for ~50% of NSAIDs
Acidic pH of the drug contributes to its quick excretion (urine is alkaline)
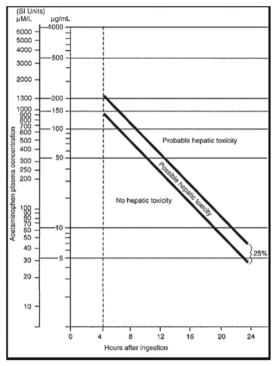
How do we treat aspirin overdose?
IV sodium bicarbonate helps make urine even more alkaline, making excretion much faster.
Less acidic, less absorption
Acetaminophen absorption
almost complete
Acetaminophen distribution
uniform throughout the body. Peak plasma concentration reached within 1h
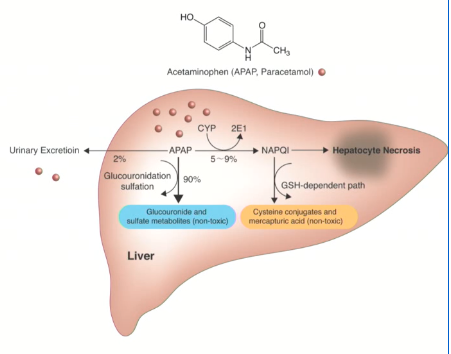
Acetaminophen Metabolism
mostly inactive conjugates in liver
small fraction:
→ NAPQI via CYP
→ detoxified by glutathione and excreted.