Quantum Numbers
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
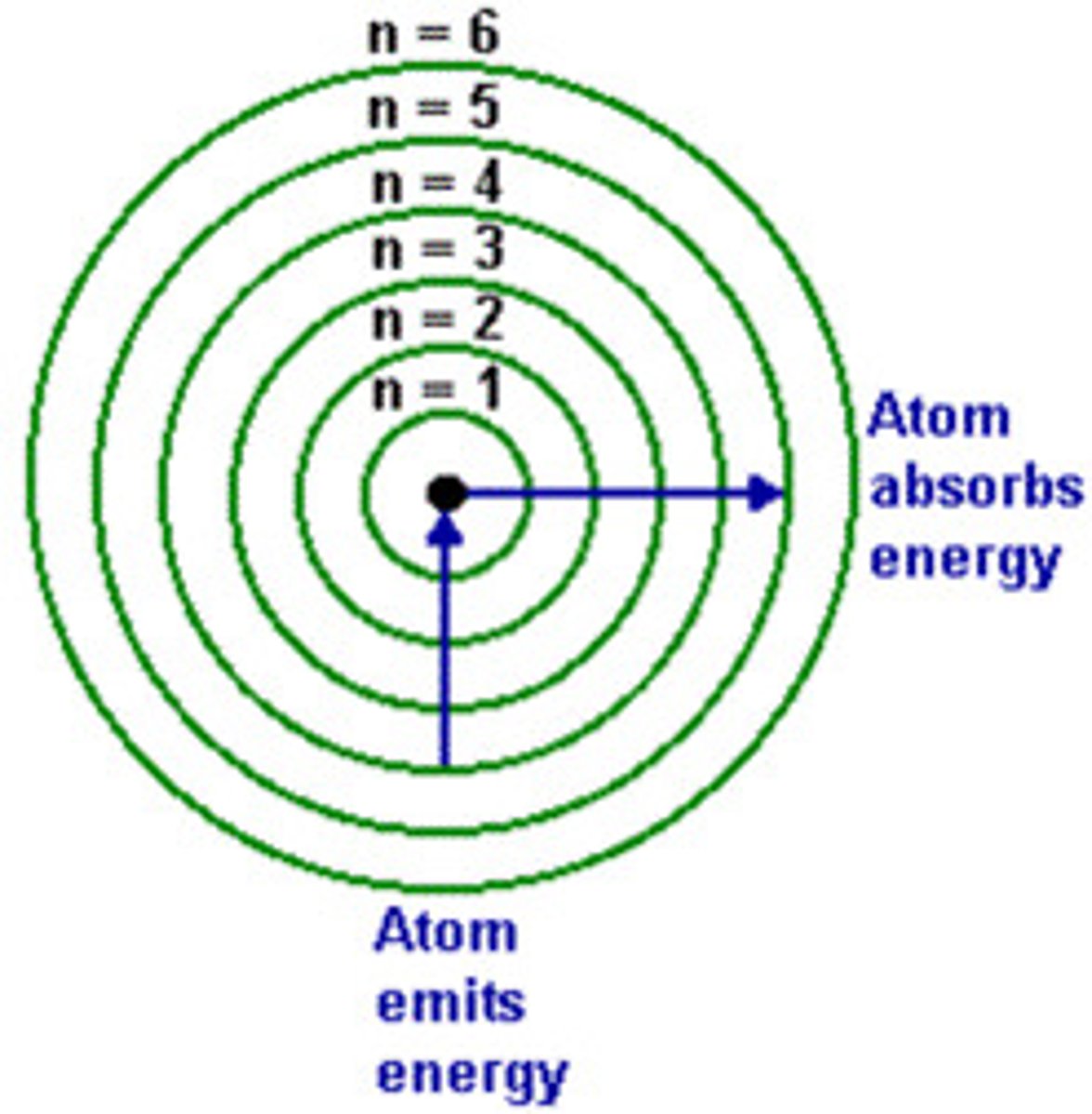
principal quantum number
symbolized by n, indicates the main energy level occupied by the electron
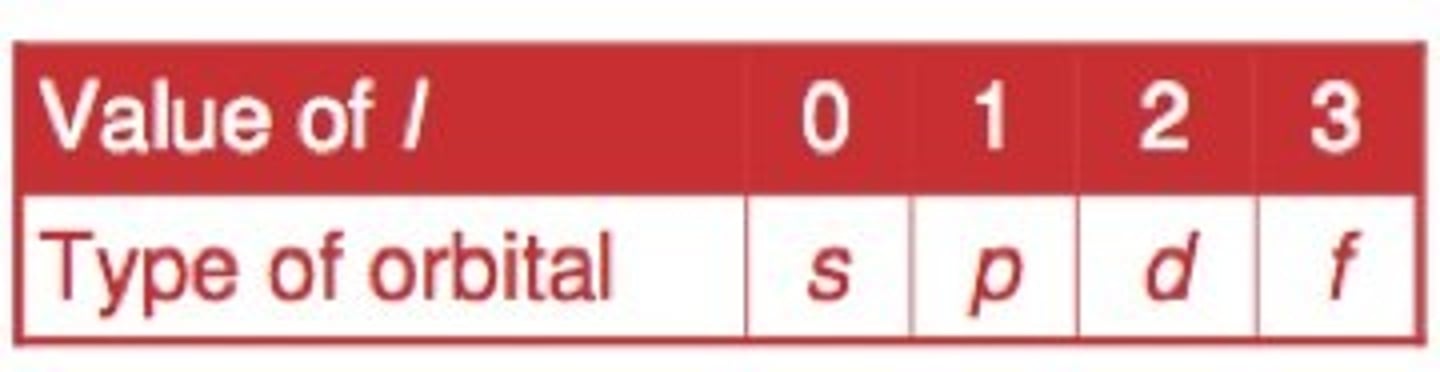
angular momentum quantum number
symbolized by l, indicates the shape of the orbital
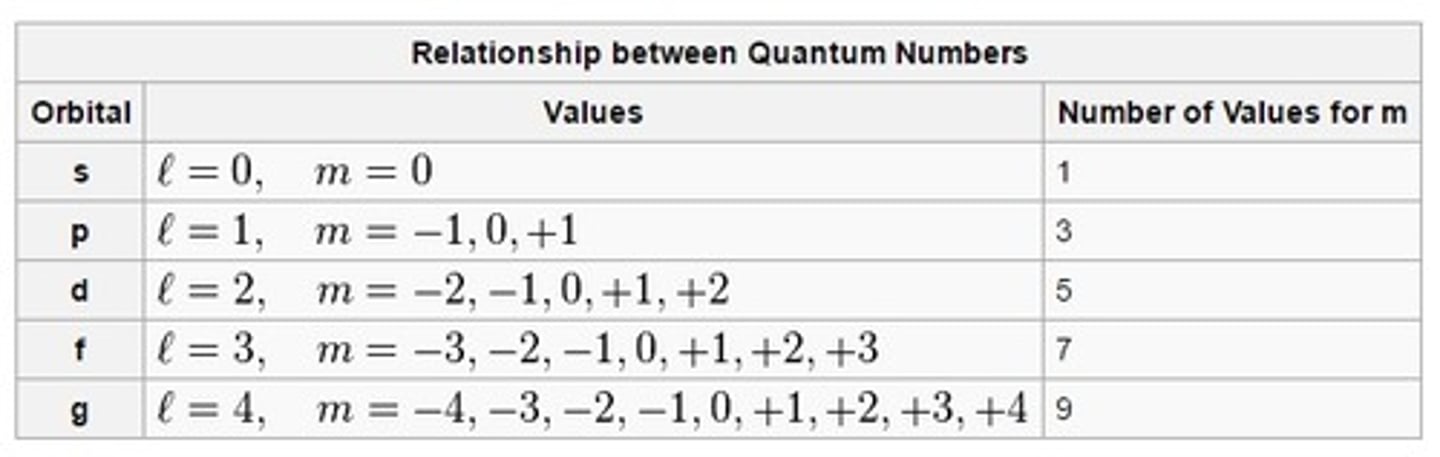
magnetic quantum number
symbolized by m, indicates the orientation of an orbital around the nucleus
spin quantum number
symbolized by s, the quantum number that has only two possible values, +1/2 and -1/2, which indicate the two fundamental spin states of an electron in an orbital
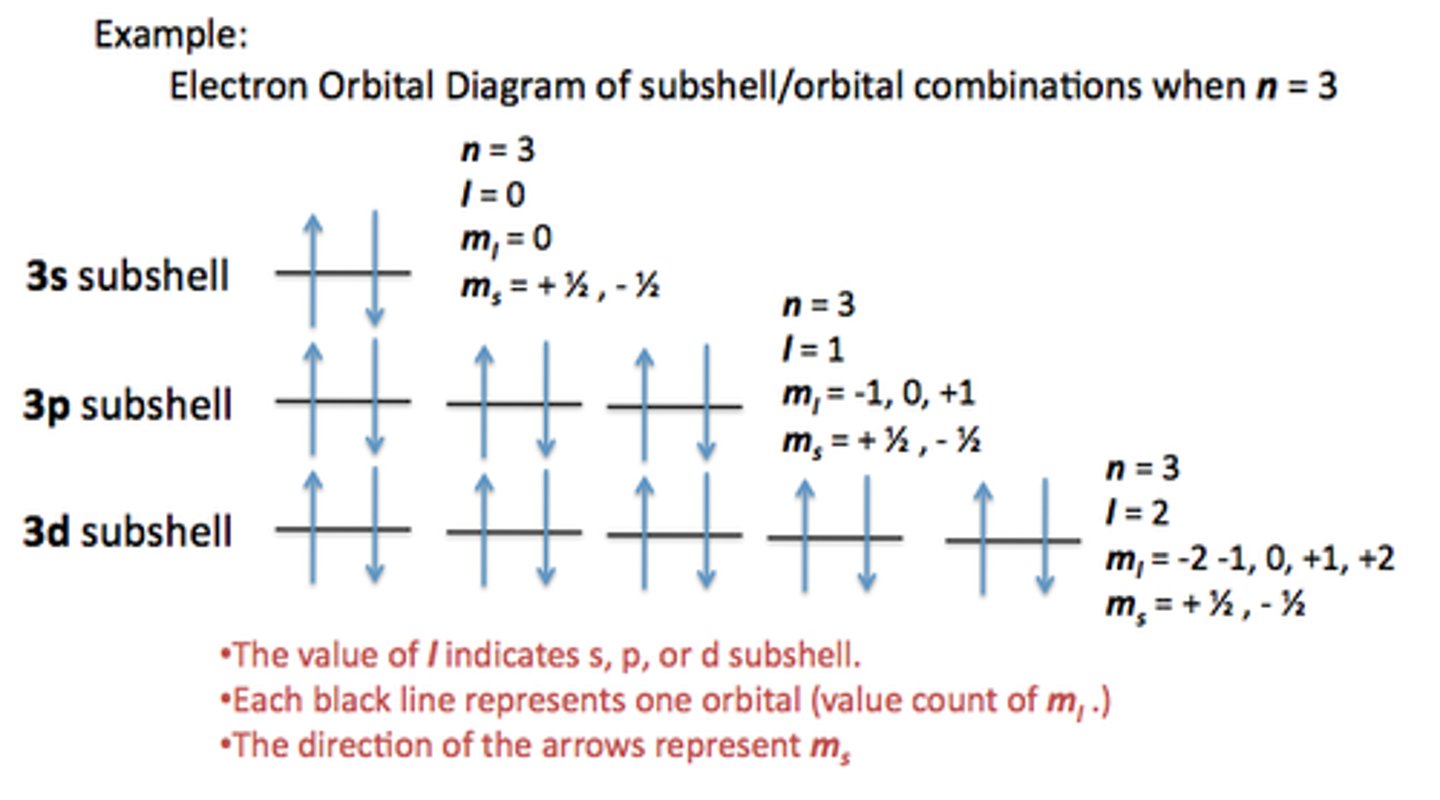
0
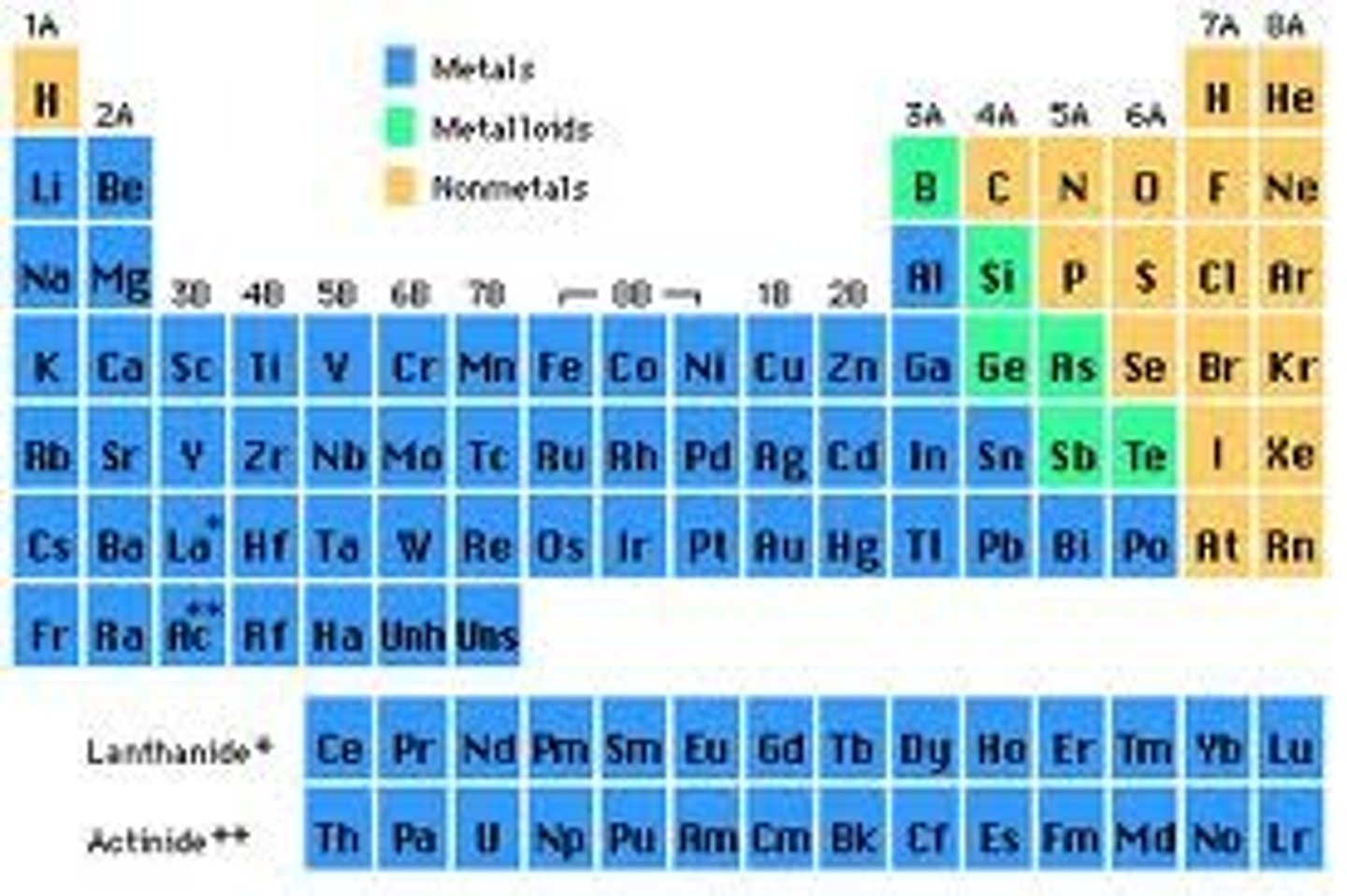
What is the angular momentum quantum (l) number if the element is in the s-block?
1
What is the angular momentum quantum (l) number if the element is in the p-block?
2
What is the angular momentum quantum (l) number if the element is in the d-block?
3
What is the angular momentum quantum (l) number if the element is in the f-block?
-1
What is the magnetic quantum number if the element has 6 valence electrons
+1
What is the magnetic quantum number if the element has 8 valence electrons
-2
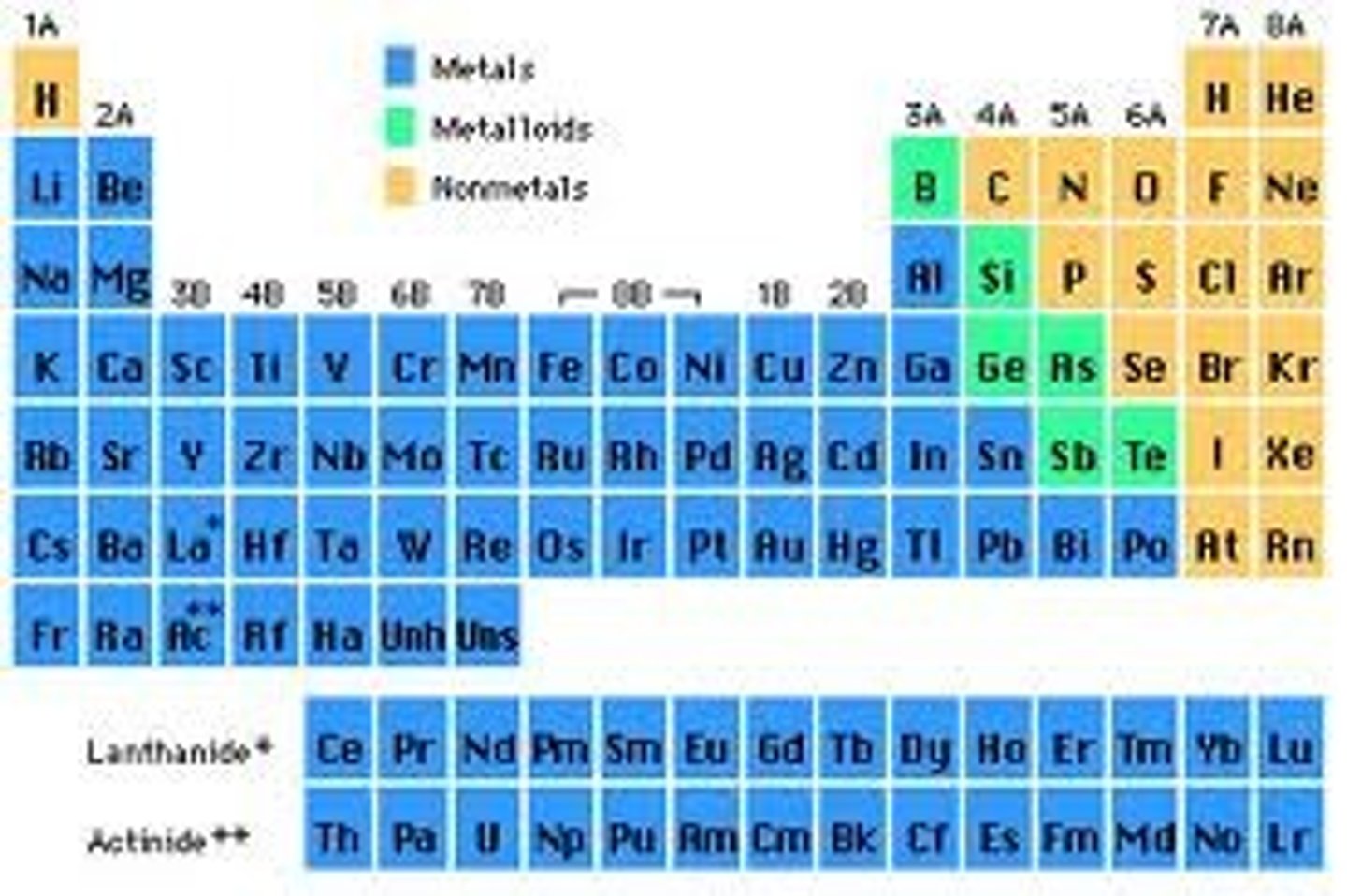
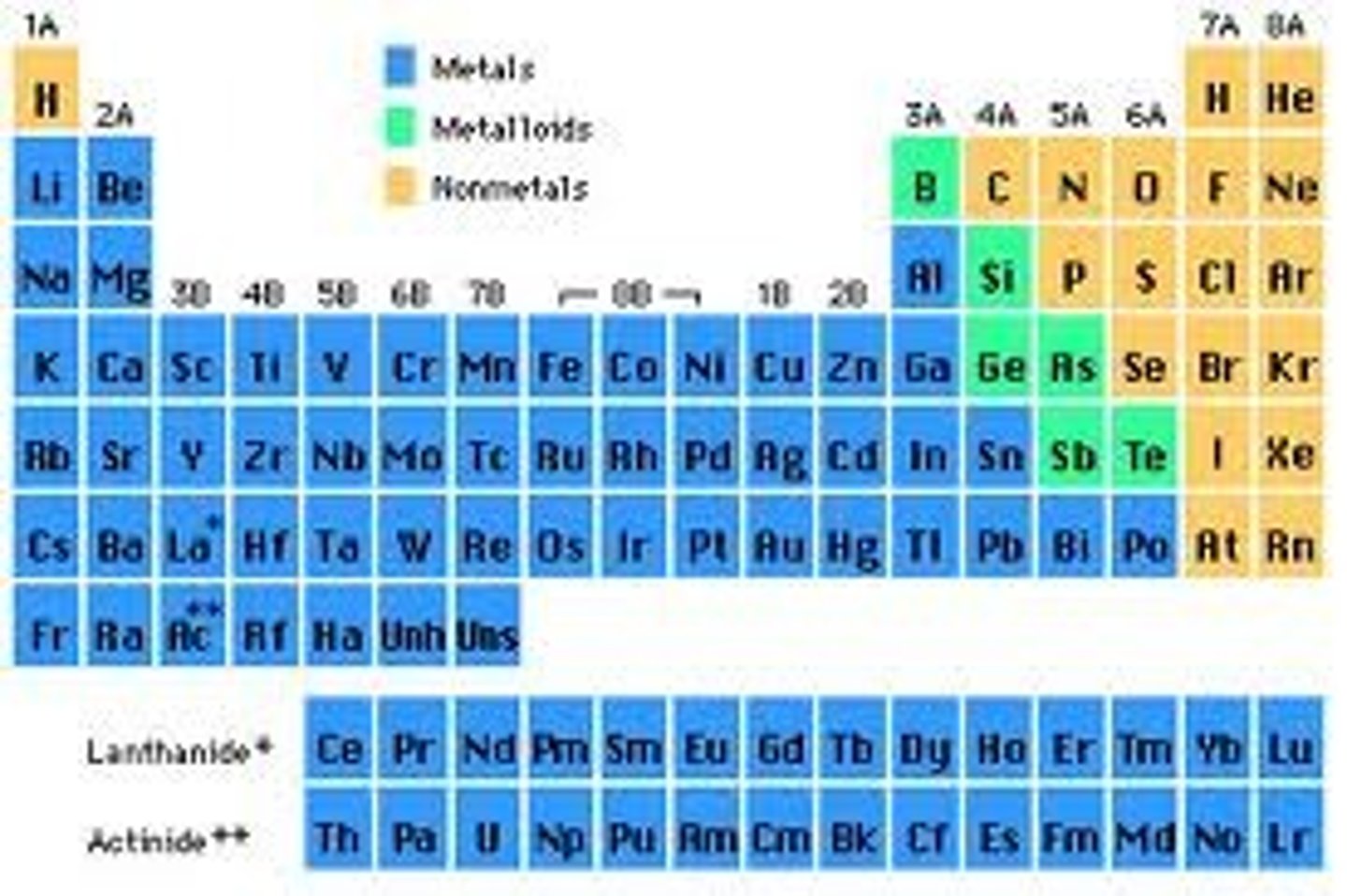
What is the magnetic quantum number if the element is Scandium (Sc)
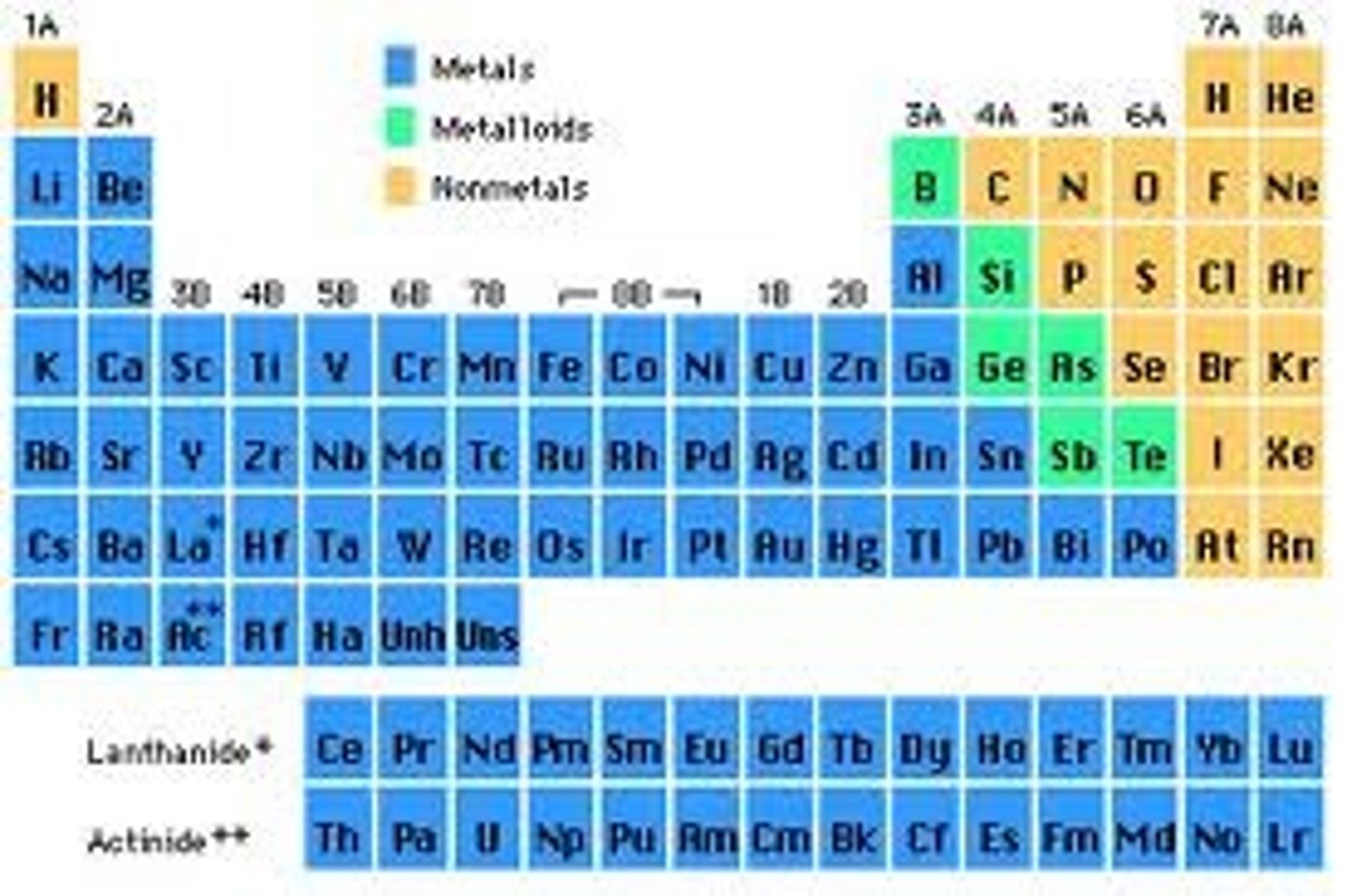
+2
What is the magnetic quantum number if the element is Manganese (Mn)
-1/2
What is the spin quantum number if the element has 8 valence electrons
+1/2
What is the spin quantum number if the element has 1 valence electrons
2, 1, -1, +1/2
Name the quantum numbers for Boron (B)
2, 1, -1, -1/2
Name the Quantum Numbers for Oxygen (O)
4, 0, 0, +1/2
Name the Quantum Numbers for Potassium (K)
4, 1, -1, -1/2
Name the Quantum Numbers for Selenium (Se)